MRD Status Is a Major Factor for Prognosis and Further Treatment in Newly Diagnosed Multiple Myeloma
Joseph Mikhael, MD, explained the prognostic factors for consideration when treating newly-diagnosed multiple myeloma.
Joseph Mikhael, MD

During a Targeted Oncology Case-Based Roundtable event, Joseph Mikhael, MD, professor, Applied Cancer Research and Drug Discovery Division, Translational Genomics Research Institute, affiliate of City of Hope, explained the prognostic factors for consideration when treating newly-diagnosed multiple myeloma.
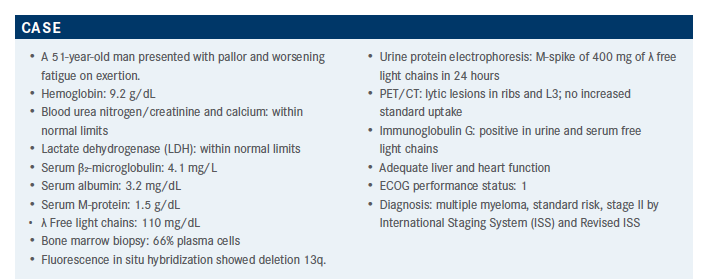
Targeted OncologyTM: What is the most accurate prognostic parameter for this patient? How does this affect your therapeutic approach?
MIKHAEL: First-line therapy and second-line therapy together are probably more prognostic than almost anything else in a patient with myeloma. The idea that we should save the best for last is not the way we’re approaching things anymore.
As for whether or not MRD [minimal residual disease] is going to be involved in our decision-making, there isn’t a single guideline that says therapy should be guided by the presence or absence of MRD negativity. However, MRD negativity is an incredibly powerful prognostic tool.1
We are trying to make the case to the FDA that MRD is a surrogate marker of PFS [progression-free survival]; this could facilitate faster drug development. I think that soon we will be making decisions based on MRD negativity, [especially since], if the highest-risk patients do not achieve MRD negativity, we know that their disease is going to be more resistant and return sooner.
By contrast, we don’t know for sure that a standard-risk patient who gets MRD negativity could stop their current therapy. We’re doing clinical trials now in which patients are brought to MRD negativity and then are randomized to continuing treatment or not or even to receiving other treatments.
The take-home message is that MRD negativity is incredibly prognostic. If it’s part of an institution standard to [assess MRD] at the day-100 [mark], I think that’s reasonable, but it would be a little premature to start making major decisions [based on MRD negativity]. Still, I think we all recognize that getting as deep a response as possible is critical, particularly in the high-risk patients.
How does MRD negativity influence transplant eligibility?
Some people [wonder whether] complete remission or even MRD negativity are necessary before transplant. We don’t have those data.
When I was at Mayo Clinic, we did this one [related] study [that showed] that people that had the best response before transplant did well in the long term. Well, there’s no shock there. However, we do know that transplant can be an equalizer. We typically want to get patients into a good depth of response prior to transplant, at least PR [partial response] if not VGPR [very good partial response]. But switching therapies 3 or 4 times to get patients into complete remission before transplant is not the right strategy.
What are the treatment options for transplant candidates?
We have many choices. According to the NCCN [National Comprehensive Cancer Network] guidelines, bortezomib [Velcade], lenalidomide [Revlimid], dexamethasone [VRd]; and bortezomib, cyclophosphamide [Cytoxan], dexamethasone [CyBorD] are the preferred regimens, and there may be situations where we use carfilzomib [Kyprolis].2
Daratumumab, lenalidomide, bortezomib, dexamethasone [dara-VRd] will likely move up [to be] one of the preferred regimens before too long. The latest version of the guidelines includes both IV daratumumab and subcutaneous daratumumab; we have 8 indications for daratumumab, of which 5 are indicated in the subcutaneous format.3
How successful are these treatments at achieving VGPR, MRD negativity, and PFS?
Studies demonstrate that we can get 75% to 90% of patients into VGPR or better, and we’re starting to see numbers where two-thirds of patients are getting to MRD negativity.4-8
The IFM 2009 study [NCT01191060] compared VRd plus or minus transplant.4 The FORTE study [NCT02203643] was 12 months of KRD [carfilzomib, lenalidomide, dexamethasone] plus or minus transplant.5 CASSIOPEIA [NCT02541383] was dara-VTd [daratumumab, bortezomib, thalidomide (Thalomid), dexamethasone] versus VTd,6 and GRIFFIN [NCT02874742] was a phase 2 randomized study of dara-VRd versus VRd.7,8
The IFM study’s 90-month follow-up showed that PFS was prolonged by a year when patients got transplant [47.3 vs 35 months; HR, 0.70; 95% CI, 0.59-0.83; P < .001].9 At the 8-year overall survival [OS], it was basically the same.
It was all a wash. There’s lots of discussion and reasons for that. In myeloma, like in any other disease, OS is more a reflection of other options later, not to mention that 79% of the nontransplant group received a transplant at first relapse.
People point to this as potential evidence for the role of potentially delaying a transplant. Although PFS was prolonged by a year [with early transplant], 8-year OS was the same [as with a delayed transplant]. MRD status was one of the most potent predictors of PFS.1,9
Are you concerned about using too many therapies in the first line and thereby leaving fewer options for future therapy?
It’s a valid concern. But as shown in several ASH [American Society of Hematology 2020 Annual Meeting] presentations, it’s better to [establish] deep response early. I’m not [suggesting] we use every available drug [as first-line therapy], but I think the principle of saving a better drug for later is probably not helpful, because it’s best to use the better drug early on. When we’ve looked at these PFS2 curves from almost all of these larger studies, [it has shown] that patient care is not compromised by using combinations earlier.
Just today I was on the phone with someone who was suggesting a 5-drug regimen to me for a clinical trial up front. I think that giving 5, 6, or 7 drugs together may [exceed the limit of how many drugs can be combined]. Even though daratumumab, for example, is not a drug that causes many of the same adverse effects as other drugs do, there’s always some overlap, and at some point, you’re going to burst the bubble.
How can the toxicity of a 4-drug regimen be mitigated?
Several different protocols are looking at quadruplet therapies, but instead of saying we’d just give this forever, it’s given until a definite response, until the patient reaches MRD negativity and then reducing it considerably when the patient no longer needs intensive therapy. That may be one of the ways of the future if we think that depth [of response] matters. That’s how we [manage] diseases like chronic myelogenous leukemia, as a good example.
How does a patient’s age influence your determination of transplant eligibility?
I led one of the ASCO [American Society of Clinical Oncology] guidelines [panels] last year, and one of the things we said explicitly in the guidelines is: Do not determine transplant eligibility by age alone.10 I like that strategy [where the age cutoff is] around 70, where if the patient is older than 70, you have to prove the case [should go] to transplant; if they are younger than 70, you have to prove the case [should not go] to transplant. That’s a reasonable guideline, but as we know, it’s a hugely heterogeneous population.
References:
1. Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456-2464. doi:10.1182/blood-2018-06-858613
2. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 4.2021. Accessed April 26, 2021. https://bit.ly/3nuC4VY
3. Giri S, Grimshaw A, Bal S, et al. Efficacy of daratumumab in the treatment of multiple myeloma with high-risk cytogenetics: meta-analysis of randomized phase III trials. J Clin Oncol. 2020;38(suppl 15):8540. doi:10.1200/JCO.2020.38.15_suppl.8540
4. Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311-1320. doi:10.1056/NEJMoa1611750
5. Gay F, Cerrato C, Petrucci MT, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: results from the FORTE trial. J Clin Oncol. 2019;37(suppl 15):8002. doi:10.1200/JCO.2019.37.15_suppl.8002
6. Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stemcell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29-38. doi:10.1016/S0140-6736(19)31240-1
7. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936-945. doi:10.1182/blood.2020005288
8. Voorhees PM, Rodriguez C, Reeves B, et al. Daratumumab (DARA) plus lenalidomide, bortezomib, and dexamethasone (RVd) in patients with transplant-eligible newly diagnosed multiple myeloma (NDMM): updated efficacy and safety analysis of the safety run-in population of GRIFFIN. Blood. 2020;136(suppl 1):3243.
9. Perrot A, Lauwers-Cances V, Cazaubiel T, et al. Early versus late autologous stem cell transplant in newly diagnosed multiple myeloma: long-term followup analysis of the IFM 2009 Trial. Blood. 2020;136(suppl 1):39. doi:10.1182/blood-2020-134538
10. Mikhael J, Ismaila N, Cheung MC, et al. Treatment of multiple myeloma: ASCO and CCO joint clinical practice guideline. J Clin Oncol. 2019;37(14):1228-1263. doi:10.1200/JCO.18.02096

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More