Ruxolitinib Continues to Show Strong Efficacy as Frontline Therapy in Myelofibrosis
The most recent data on ruxolitinib support its continued use as frontline treatment of patients with myelofibrosis.
During a Targeted Oncology Case-Based Roundtable event, Haris Ali, MD, associate clinical professor, Department of Hematology and Hematopoietic Cell Transplantation at City of Hope, discussed the most recent data supporting the use of ruxolitinib (Jakafi) as frontline treatment of patients with myelofibrosis (MF).
Targeted OncologyTM: What prognostic tools would be used for this patient?
ALI: The 3 most commonly used prognostic scoring systems are IPSS [International Prostate Symptom Score], DIPSS [Dynamic International Prognostic Scoring System], and DIPSS-Plus. IPSS and DIPSS are the same except the hemoglobin [level] gets 2 points in the DIPSS; DIPSS also added karyotype transfusion requirements and low platelet count.1 Generally, in low-risk groups the prognosis for survival is very good, with a median overall survival [OS] anywhere from 11 years to 15 years.…High-risk patient survival, [on the other hand,] is 1 to 2 years, and intermediate is somewhere in between those [2 ranges].
How do gene mutations factor into this patient's prognosis?
Patients with ASXL1, EZH2, SRSF2, or IDH1/2 [mutations in their disease] are high risk, and all of them are associated with high risk for leukemic transformation, additionally. The higher the number of these HMR [high-risk molecular] changes, the greater the risk for leukemic transformation, disease progression, and decreased survival. [In this case, the] MIPSS70 score would be used.2 However, this is really good with the median OS of about 27 years compared with intermediate risk, which is 7 years; and high risk is about 2.3 years.
Can you discuss the newest prognostic tool, Mutation-Enhanced International Prognostic Scoring System (MIPSS70)?
[There is also] a new classification for karyotype that further refined the prognostic groups. In this, the very low–risk group was the people who did very well, with a 92% and 80% chance of 10-year survival [and a median survival that was not reached] compared with the very high–risk group, [in which] the median OS was only 1.8 years.3 Very high–risk karyotype included the deletion 7, isochromosome q, inv(3)/3q21, deletion 11q, and some other trisomies, excluding the trisomies 8 and 9. Moreover, U2AF1 Q157is also not included as an HMR for one of the mutations that lowers the survival and leukemic transformation risk. leukemic transformation risk.
There is a new prognostic scoring system that was specific for secondary myelofibrosis that’s post polycythemia vera and post essential thrombocythemia. They had similar variables, including age, hemoglobin, platelets, blasts, CALR mutations, and constitutional symptoms.4 They have similar risk groups: high, intermediate-2, intermediate- 1, and low-risk. OS for the low-risk [group] did not reach 14 years, and for intermediate-1 it was 10 years. Intermediate-2 was closer to 4 years, and for the high-risk group it was about 2.2 to 2.5 years.
What are the prognostic guidelines for a patient like this?
Currently, the National Comprehensive Cancer Network guidelines recommend using any one of these prognostic scoring systems.5 You can get good information about [the patient’s] risk group. If you have all the data available, you can use the MIPSS70-Plus. If not, then you can use IPSS or DIPSS-Plus if you have the cytogenetics, which get divided between the low-risk and high-risk categories.
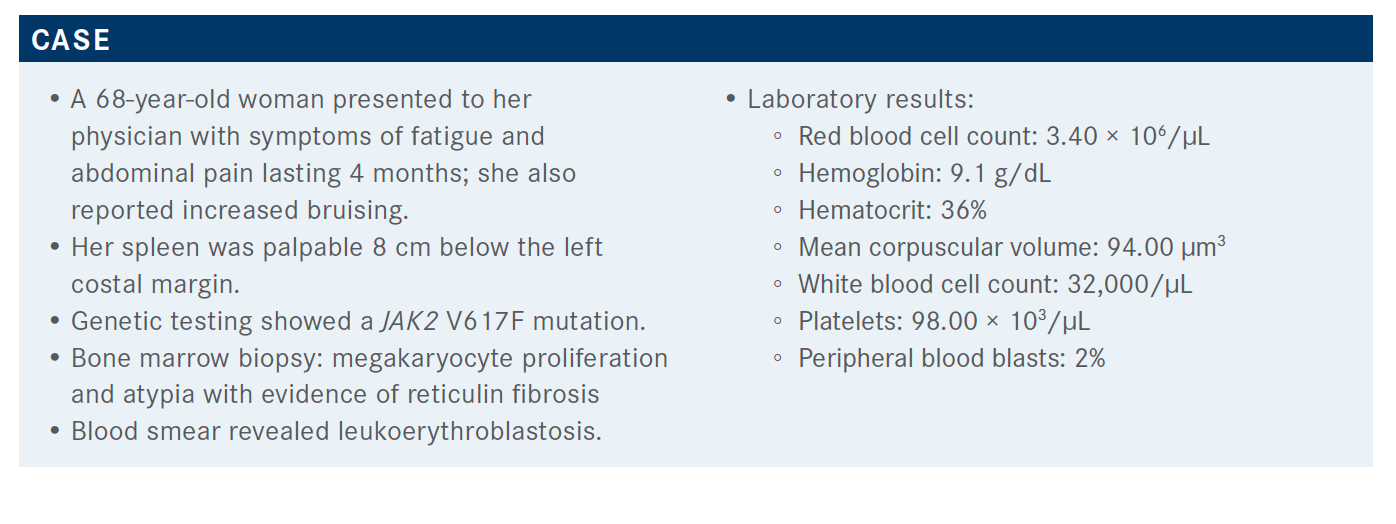
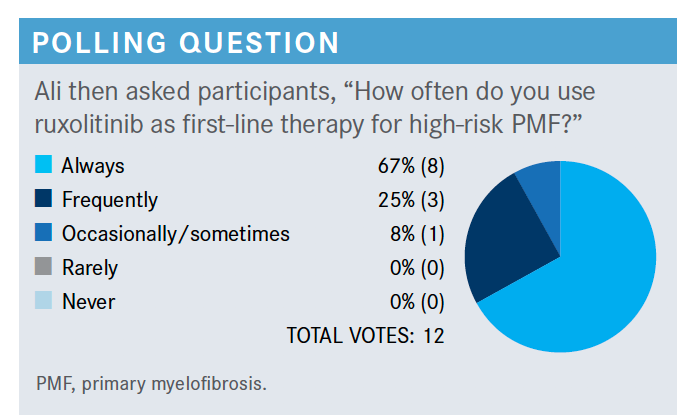
Which trials led to the approval of ruxolitinib (Jakafi) for this patient population?
They were the COMFORT-I [NCT00952289] and COMFORT-II [NCT00934544] trials. The COMFORT-I trial was primarily in the United States and COMFORT-II was primarily in Europe. In the COMFORT-I trial, they compared ruxolitinib with placebo, whereas in COMFORT-II they compared ruxolitinib with a 2:1 ratio to the best available treatment.6,7
The primary end point [in both studies] was 35% spleen volume reduction at 34 weeks. In COMFORT-I, that was almost 42% of the patients who had a spleen volume reduction. 6 [In comparison, patients on the] COMFORT-II trial had a 28% spleen volume reduction.7 Pretty much all the patients had spleen volume reduction, about 95% who were on ruxolitinib compared with placebo [in COMFORT-I].6
Very similar results were seen for the COMFORT-II trial, and most of the patients on ruxolitinib had spleen volume reduction. About 97% of the patients had spleen volume reduction [with ruxolitinib] compared with 56% [on best available therapy], and the increase in the spleen volume was 3% on ruxolitinib versus 44% in the best available therapy arm.7
How did safety factor into the outcomes of this study?
The secondary end point was improvement in the constitutional symptoms. The majority of the patients who were on ruxolitinib [in the COMFORT-I study] had symptom reduction.6 More than 50% reduction was seen in quite a lot of patients compared with the placebo arm. Basically, all of the symptoms improved to some degree, and that response started to appear as soon as the patient started treatment, and the patient had pretty much maximal response between 4 and 8 weeks in terms of symptom improvement.
In COMFORT-II, researchers recorded symptom improvement through European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire core model scores.7 Improvement was shown by positive numbers, and there was a reduction in symptoms. Clearly, when these trials were conducted, ruxolitinib was the best treatment compared with any of the best available therapy arms.8 [Regarding adverse events (AEs)] in COMFORT-II, about 40% of the patients had anemia, about 8% to 13% had thrombocytopenia, and about 7% had neutropenia.
What were the survival data in these trials?
Researchers combined the patients in COMFORT-I and COMFORT-II and looked at the OS. The median OS was 5.3 years [95% CI, 4.7-not estimable] in the ruxolitinib arm, which was much greater than the control arm of 3.8 years [95% CI, 3.2-4.5].9 The cause of death among the patients who died was either transformation to acute leukemia or myelofibrosis progression without acute leukemia, thrombosis, or cardiovascular events, such as infection, bleeding, portal hypertension, and other causes.9
How does spleen response affect the outcome of ruxolitinib-treated patients?
The patients who had a spleen response at 6 months had a significantly higher OS compared with patients who did not have a spleen response by 6 months.10 Moreover, the durability of response was also significant with OS in patients who had a reduction of treatment [due to their spleen response].
Of those who were on less than 10 mg [of ruxolitinib] twice a day, only 18% met the primary end point, which was 35% volume reduction, compared with [those who were on a] higher dose.11 So there were much better responses at a higher dose level, starting at 10 mg twice-daily dosing and beyond. The main takeaway from this was avoiding starting at a low dose, then escalating, because the higher the dose is started at, the better the responses you get in these patients.
How does platelet count affect your decision about the ruxolitinib dose?
[In 1 analysis of platelet counts] they had 2 strata: stratum 1, with platelet count a little bit better at 75,000 to 99,000 mL, versus stratum 2, which was 50,000 to 74,000 mL.12 They gradually escalated the dose in this patient with 55,000 to 99,000 platelets, from 5 mg twice daily all the way up to 15 mg twice daily. Once they found out that 10 mg twice daily was a safe dose, then they started stratum 2, which was 50,000 mL—much lower platelets. They noticed that for both of these groups, the maximum safe starting dose was 10 mg twice daily.
What effect does age have on treatment decisions for this population?
If they are 75 years or older, I’m OK starting them on 10 mg twice daily. I used to start them on 5 mg twice daily, but [these data] were pretty encouraging. You can start these patients on 10 mg twice daily and watch them closely. If you continue it in a good proportion of patients without too many AEs, you get better spleen response and symptom response.
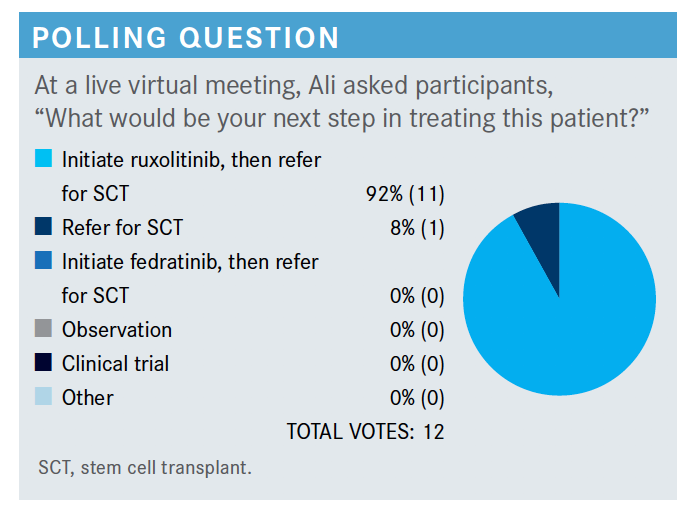
Are there any other options for treatment in these patients?
We have another option, another JAK inhibitor, fedratinib [Inrebic]. That was approved about 2 years ago, both in the up-front setting and also in the setting of patients who have failed ruxolitinib, based on 2 trials, JAKARTA and JAKARTA2 [NCT01437787 and NCT01523171].13 The original JAKARTA trial was [with patients with a new diagnosis of] intermediate-2 and high-risk disease. They were divided into 3 arms: placebo, fedratinib 400 mg, and fedratinib 500 mg. The FDA approved the drug at 400-mg dosing.
In patients on the JAKARTA trial, about 36%…had a spleen response, and the higher the platelet count, the more spleen responses.11 For example, [in patients] with more than 100,000 platelets, 49%…had a spleen response and 42% had symptom response versus patients with low platelets, who had 36% spleen response and 31% symptom reduction. The majority of the patients on fedratinib had response compared with the patients on placebo, who had a spleen reduction at 34 weeks.
Were there any AEs associated with fedratinib for patients with myelofibrosis?
There is a black box warning for some of the patients, and the trial was put on hold. There was a total of 8 patients who were on the drug who experienced encephalopathy, 1 case of Wernicke encephalopathy, and…other suspicious cases of encephalopathy. Now, these patients were tested for thiamine level before treatment and throughout the treatment and given a thiamine supplement to prevent any risk for encephalopathy while on treatment. I have treated a lot of patients on fedratinib. Personally, I have not seen anyone with encephalopathy. All of them are on the thiamine supplement.
When should patients such as these be considered for transplant?
You should consider them for transplant if they’re young and they could have a donor, so proceed to transplant if there’s an option. If they are not a transplant candidate, you assess burden. If the platelet count is less than 50,000 mL, they can be referred for clinical trials.5 If it is more than 50,000 mL, you can use the ruxolitinib or fedratinib again or you can always use a clinical trial. Monitor responses and as long as it is working, you continue treatment. If they progress to acute myeloid leukemia, then the treatment paradigm changes completely. If someone is not a candidate for transplant and they have anemia only, then [use] treatment directed toward anemia, which could be either growth factors or other agents that can help with anemia.
References:
1. Masarova L, Bose P, Pemmaraju N, et al. Prognostic value of blasts in peripheral blood in myelofibrosis in the ruxolitinib era. Cancer. 2020;126(19):4322-4331. doi:10.1002/cncr.33094
2. Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310-318. doi:10.1200/JCO.2017.76.4886
3. Tefferi A, Nicolosi M, Mudireddy M, et al. Revised cytogenetic risk stratification in primary myelofibrosis: analysis based on 1002 informative patients. Leukemia. 2018;32(5):1189-1199. doi:10.1038/s41375-018-0018-z
4. Tefferi A, Guglielmelli P, Lasho TL, et al. MIPSS70+ version 2.0: Mutation and Karyotype-Enhanced International Prognostic Scoring System for primary myelofibrosis. J Clin Oncol. 2018;36(17):1769-1770. doi:10.1200/JCO.2018.78.9867
5. Passamonti F, Giorgino T, Mora B, et al. A clinical-molecular prognostic model to predict survival in patients with post polycythemia vera and post essential thrombocythemia myelofibrosis. Leukemia. 2017;31(12):2726-2731. doi:10.1038/leu.2017.169
6. NCCN. Clinical Practice Guidelines in Oncology. Myeloproliferative neoplasms, version 1.2021. Accessed April 22, 2021. https://bit.ly/2Wcczfa
7. Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi:10.1056/NEJMoa1110557
8. Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787-798. doi:10.1056/NEJMoa1110556
9. Verstovsek S, Gotlib J, Mesa RA, et al. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J Hematol Oncol. 2017;10(1):156. doi:10.1186/s13045-017-0527-7
10. Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895-2901. doi:10.1182/blood-2008-07-170449
11. Pardanani A, Harrison C, Cortes JE, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 2015;1(5):643-651. doi:10.1001/jamaoncol.2015.1590
12. Vannucchi AM, Te Boekhorst PAW, Harrison CN, et al. EXPAND, a dosefinding study of ruxolitinib in patients with myelofibrosis and low platelet counts: 48-week follow-up analysis. Haematologica. 2019;104(5):947-954. doi:10.3324/haematol.2018.204602
13. FDA approves fedratinib for myelofibrosis. FDA. August 16, 2019. Accessed April 22, 2021. https://bit.ly/3dErcBr
