BTK Inhibitors Provide Extensive Frontline Treatment Options in Treatment-Naïve CLL
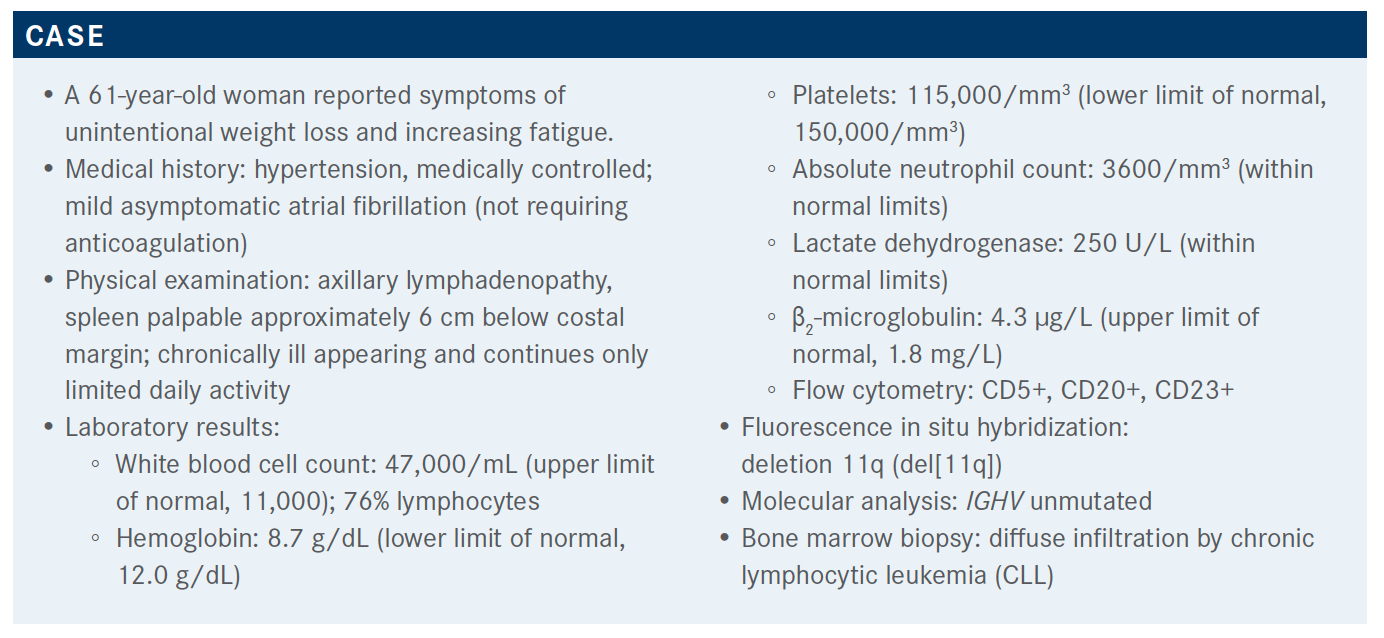
Seema A. Bhat, MD, discussed the case of 61-year-old patient with chronic lymphocytic leukemia during a Targeted Oncology Case-Based Roundtable event.

Seema A. Bhat, MD, assistant professor of Medicine, Division of Hematology, The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, discussed the case of 61-year-old patient with chronic lymphocytic leukemia (CLL) during a Targeted Oncology Case-Based Roundtable event.
Targeted OncologyTM: What is the current standard frontline therapy for patients with CLL?
BHAT: We will review some of the data in decision-making for patients with CLL who require frontline treatment. We will start with the RESONATE-2 study [NCT01722487], which was a frontline ibrutinib [Imbruvica] study.1 This study included older patients with CLL who required treatment per iwCLL [International Workshop on Chronic Lymphocytic Leukemia] guidelines criteria. This study excluded patients with del(17p). Patients were randomized between the standard-of-care arm with single-agent chlorambucil for 12 cycles and the experimental arm with ibrutinib, given at "420 mg/day until disease progression.
There were 55 patients who crossed over to the single-agent ibrutinib arm upon disease progression. The primary end point was progression-free survival [PFS]. The primary analysis was presented at 18.4 months’ follow-up, which led to the approval of ibrutinib. The median PFS was not reached at that time for ibrutinib, and the median PFS for chlorambucil at that point was 18.9 months. We have a 5-year follow-up where the median for ibrutinib is not reached, whereas for chlorambucil it is 15 months. More than 50% of the patients continue to be on the ibrutinib arm at this point at the 5-year follow-up.2
Is a Bruton tyrosine kinase (BTK) inhibitor standard for all patients? For all high-risk patients?
There is a difference in [PFS between] high-risk groups [based on] IGHV status and del(11q). Remember, patients with del(17p) were not included. The poor prognosis of IGHV unmutated status is pretty much eliminated by ibrutinib. The [PFS] curves by [IGHV status] are pretty much superimposing, meaning that ibrutinib is almost equally effective in IGHV-mutated and -unmutated cases. Patients with [or without] del(11q) [on ibrutinib] do much better compared with single-agent chlorambucil.
One can argue that for the RESONATE-2 [regimen], nobody uses chlorambucil, so this comparison is not valid. We did have the CLL10 study [NCT00769522] data at that point where FCR [fludarabine, cyclophosphamide, and rituximab (Rituxan)] was [compared] head-to-head with BR [bendamustine, rituximab].3 So, at that point, FCR was the standard of care.
Is it better to add a CD20-targeted antibody like rituximab to a BTK inhibitor?
The cooperative group study did a head-to-head comparison between ibrutinib and the CLL10 regimen in separate studies. The ECOG1912 study [NCT02048813] was performed in patients younger than 70 years.4 Again, patients with del(17p) were excluded, and patients were randomized between receiving FCR in the standard fashion versus the experimental arm, which included ibrutinib plus rituximab where rituximab was given for 6 cycles. The primary end point was PFS.
The PFS at 3 years was 89% for the ibrutinib-plus-rituximab arm versus 71% for the FCR arm, which was highly statistically significant. Also, significant is the 3-year overall survival [OS] rate: 98.8% for the ibrutinib-containing arm versus 91.5% for the FCR arm. Seeing the survival benefit at 3 years in an up-front study in CLL is remarkable.
There is a difference [in PFS] between patients with IGHV unmutated [and mutated status]. Ibrutinib did far better compared with FCR in patients with IGHV unmutated status [HR, 0.28; 95% CI, 0.17-0.48; P < .0001].5
Which BTK inhibitor do you use and how do you select it?
The targeted agent is acalabrutinib [Calquence], a more selective next-generation BTK inhibitor. Acalabrutinib does not have some of the effects on some targets that possibly explain the adverse effects [AEs] of ibrutinib. We wanted to see how ibrutinib compared with standard treatment.
Elevate CLL TN [NCT02475681] was a global multicenter study that accrued 535 treatment-naive patients requiring treatment.6 Patients were mostly older than 65, but patients younger than 65 were allowed on the study if they had some comorbid disease with a high CIRS [Cumulative Illness Rating Scale] score or a low creatinine clearance. These patients were randomized to receive obinutuzumab [Gazyva] plus chlorambucil for 6 cycles, single-agent acalabrutinib, or acalabrutinib plus obinutuzumab where obinutuzumab was given for 6 cycles and acalabrutinib was continued indefinitely.
The primary end point was PFS difference between the 2 obinutuzumab-containing arms [acalabrutinib plus obinutuzumab versus obinutuzumab plus chlorambucil]. The secondary end point included PFS difference between acalabrutinib versus obinutuzumab/chlorambucil, overall response rate [ORR], safety, and OS. Crossover was allowed from the obinutuzumab/chlorambucil [arm] to the single-agent acalabrutinib arm based on independent review committee assessment and confirmed disease progression.
What was the efficacy of Elevate CLL TN?
At a median follow-up of 28.3 months, the median PFS was not reached for the 2 acalabrutinib arms, whereas it was 22.6 months for chlorambucil plus obinutuzumab, which was highly statistically significant.6
The PFS [was categorized] by different subgroups, including age, Rai stage, ECOG performance status, bulky disease, and risk factors. [On the forest plot], acalabrutinib did much better than chlorambucil plus obinutuzumab in all risk categories, including high risk. The patients with del(11q) and IGHV mutated versus unmutated or del(17p) patients all responded much better to the acalabrutinib.
The ORR was about 94% with acalabrutinib plus obinutuzumab, 85% with single-agent acalabrutinib, and only 79% in the obinutuzumab/chlorambucil arm, demonstrating the benefit of obinutuzumab. Unfortunately, in this study, the difference between obinutuzumab single agent versus acalabrutinib/obinutuzumab was a post hoc analysis of PFS. This was a missed opportunity to see a difference between these 2 arms, but for the ORR, the CR [complete response] rates are higher and 13% of the patients obtained a CR in the acalabrutinib-plus-obinutuzumab arm, whereas in the single-agent acalabrutinib arm, only 1 patient had a CR.6
If you compare the data from this study with long-term data from patients who have been on single-agent ibrutinib, CR rates continue to increase. At 1 year they were around 10%, and at 5 years, 30% of the patients had a CR with continued use of ibrutinib. This study needs a longer follow-up, to see whether more patients in the single-agent ibrutinib arm will convert to a CR.6
OS was one of the secondary end points. There is separation of the [OS] curves [for acalabrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab] [HR, 0.47; 95% CI, 0.21-1.06; P = .0577], which is interesting. It is a short follow-up and seeing such a difference at a short follow-up is highly encouraging. Acalabrutinib, both for PFS and OS, outdoes the chlorambucil-plusobinutuzumab arm.7
Please discuss the toxicity profile seen in this trial.
The most common AEs [for patients treated with acalabrutinib] seen were anemia, neutropenia, upper respiratory tract infection, thrombocytopenia, headache, diarrhea, and musculoskeletal pain.
The AEs of clinical interest were AF [atrial fibrillation]. and hypertension. AF of any grade was seen in about 3% to 4% of the patients, but grade 3 or higher AF was less than 1%. The same was true for hypertension, which was seen in about 4% to 7% of the patients, but grade 2 was close to about 3%. Major bleeding was seen in less than 2% of the patients. Rate of infections was higher in the acalabrutinib-containing arms, but with the chlorambucil/obinutuzumab arm, the AE reporting period was only 6 to 7 months compared with acalabrutinib, which is an ongoing treatment; hence, reporting [of AEs during] treatment is also extended.7
I want to talk about one of the AEs that can happen after initiating acalabrutinib, and that is headache. I have learned through experience to warn my patients about this because if I do not warn them, patients get alarmed and we get called in the clinic, [with questions such as] “What’s happening to me? Am I having a stroke?” We have observed this, and it has been reported in the clinical trials also. Patients can get quite a significant headache, [which] usually happens in the initial cycle; however, this headache is very [brief] and goes away after the first couple of cycles. We treat it with caffeine and they do very well. If you support these patients through the headache, using the appropriate treatment, it usually does not recur.
What other agents are available in this setting?
Another targeted agent that we haven’t discussed much yet is venetoclax [Venclexta]. The indication for venetoclax up front comes from the phase 3 study done by the German CLL Study Group, called the CLL14 trial [NCT02242942], which compared a fixed-duration venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab. 8 This study also included up-front patients who were not very fit; venetoclax was given for 1 year and obinutuzumab was added for the first 6 months.
Chlorambucil was given for 12 cycles and obinutuzumab was added for the first 6 months, for 6 cycles of treatment. The primary end point was investigator-assessed PFS, and this study looked very closely at MRD [minimal residual disease] as a secondary end point.9
Based on the data from this study, the median PFS for venetoclax plus obinutuzumab was not reached, and for chlorambucil/obinutuzumab it was 36.4 months. Four-year PFS was much better for venetoclax plus obinutuzumab [at] 74% versus 35% with chlorambucil/obinutuzumab. Four-year time to next treatment was 81.08% with venetoclax plus obinutuzumab versus 56% for chlorambucil.
I want to highlight [something] I use in my own clinical practice for decision-making: [PFS for] the high-risk patients, IGHV mutated versus unmutated, TP53 status mutated versus unmutated. Venetoclax plus obinutuzumab does much better [than chlorambucil and obinutuzumab], but there is a separation [in the PFS curves] between the IGHV-unmutated [population] compared with the IGHV mutated. There is a slight difference between IGHV-mutated versus -unmutated response, with IGHV mutated doing better with venetoclax plus obinutuzumab.10
Similarly, for TP53 status, those without the TP53 mutation do better compared with the ones who do have TP53 mutation. I use these data for my clinical decision-making. I still prefer [to use] a BTK inhibitor in high-risk patients based on the evolving data from the CLL14 trial.
Would that mean that this therapy should be given for more than 1 year because the curves separate later?
This was looked at in the MURANO study [NCT02005471], which included patients with relapsed/refractory disease treated with venetoclax plus rituximab.11 They did see that the patients who had high-risk features [including] IGHV unmutated, complex karyotype or TP53 mutations were difficult to get into MRD-negative status, which correlates with a better PFS. These [features] tend to show that they are high risk and thus may not be the right candidates [for this regimen]. We’ll see [this] in future studies for time-limited therapy.
[The CLL14] trial also looked at the MRD data both in the peripheral blood and the bone marrow, and you can see both for the peripheral blood and the bone marrow, venetoclax plus obinutuzumab caused a higher percentage of undetectable MRD compared with chlorambucil. In the peripheral blood, [the undetectable MRD rate] was 76% with venetoclax plus obinutuzumab compared with 35% with chlorambucil/obinutuzumab. In the bone marrow, [rates were] 57% versus 17%, respectively.

What will MRD testing standards be in real-world practice? Any ideas about that?
I think it’s an evolving and interesting concept, but I think MRD testing should be limited to clinical trials at this time, [and] as the data mature, we will be able to develop more guidelines to use it in clinical practice.
For the differences in AEs [for venetoclax/obinutuzumab and chlorambucil/obinutuzumab], the hematological AEs, such as neutropenia, thrombocytopenia, and febrile neutropenia were similar. Infusion reactions were similar between the 2 groups, as were metabolic and nutritional disorders, especially tumor lysis syndrome. Interestingly, tumor lysis syndrome was mostly biochemical. None of the patients had [the criteria for] clinical tumor lysis, but tumor lysis syndrome was seen in 3 patients with venetoclax/obinutuzumab. This was in a clinical trial setting. It may be different in our practice setting.

References:
1. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437. doi:10.1056/NEJMoa1509388
2. Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of firstline ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787-798. doi:10.1038/s41375-019-0602-x
3. Eichhorst B, Fink AM, Bahlo J, et al; German CLL Study Group (GCLLSG). First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928-942. doi:10.1016/S1470-2045(16)30051-1
4. Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432-443. doi:10.1056/NEJMoa1817073
5. Shanafelt TD, Wang V, Kay NE, et al. Ibrutinib and rituximab provides superior clinical outcome compared to FCR in younger patients with chronic lymphocytic leukemia (CLL): extended follow-up from the E1912 Trial. Blood. 2019;134(suppl 1):33. doi:10.1182/blood-2019-126824
6. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278-1291. doi:10.1016/S0140-6736(20)30262-2
7. Sharman JP, Banerji V, Fogliatto LM, et al. ELEVATE TN: Phase 3 study of acalabrutinib combined with obinutuzumab (O) or alone vs O plus chlorambucil (Clb) in patients (Pts) with treatment-naive chronic lymphocytic leukemia (CLL). Blood. 2019;134(suppl 1):31. doi:10.1182/blood-2019-128404
8. Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225‐2236. doi:10.1056/NEJMoa1815281
9. Al-Sawaf O, Zhang C, Tandon M, et al. Fixed-duration venetoclax-obinutuzumab for previously untreated patients with chronic lymphocytic leukemia: follow-up of efficacy and safety results from the multicenter, open-label, randomized, phase III CLL14 trial. J Clin Oncol. 2020;38(suppl 15):8027. doi:10.1200/JCO.2020.38.15_suppl.8027
10. Fischer K, Ritgen M, Al-Sawaf O, et al. Quantitative analysis of minimal residual disease (MRD) shows high rates of undetectable MRD after fixed-duration chemotherapy-free treatment and serves as surrogate marker for progression-free survival: a prospective analysis of the randomized CLL14 trial. Blood. 2019;134(suppl 1):36. doi:10.1182/blood-2019-125825
11. Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax–rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107-1120. doi:10.1056/NEJMoa1713976

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More