Selpercatinib Shows Promise as Valuable Treatment Option for Patients With RET-Mutated Thyroid Cancer
During a Targeted Oncology Case-Based Roundtable event, Krzysztof Misiukiewicz, MD, discussed the case of a 58-year-old patient with RET-mutated thyroid cancer
Krzysztof Misiukiewicz, MD

During a Targeted Oncology Case-Based Roundtable event, Krzysztof Misiukiewicz, MD, associate professor, Medicine, Hematology and Medical Oncology and assistant professor, OtolaryngologyClinical Director, Research in Head and Neck Clinical Director, Center for Personalized Cancer Therapeutics in The Tisch Cancer Institute at The Icahn School of Medicine at Mount Sinai, discussed the case of a 58-year-old patient with RET-mutated thyroid cancer.

Targeted OncologyTM: What additional workup would you order?
BHANDARI: This patient will need a whole workup, and genetic screening and molecular testing —obviously molecular testing to look at mutations only if it’s a volumetric static disease. Obviously, these patients would undergo surgical resection, and followed by [radioactive iodine therapy].
MISIUKIEWICZ: Absolutely. Maybe the genetic screening is not important for the therapeutic purposes, but it can be considered in the patient with the germline mutation because these patients can have family so genetic counseling can be important. Having the information from this patient and the germline mutation test can be obviously important. One time, I had a patient who came with medullary thyroid cancer, and there was a medical record saying the patient was RET negative. But because they only checked for the germline mutation, they didn’t really analyze the tumor.
When they analyzed the tissue, the patient did have a mutation. So obviously, you have to be very, very open-minded, and pay close attention to details. But absolutely, that’s correct, I would do the same thing.
Would you recommend systemic treatment for this patient now? If yes, why? If not, why? And would you order any mutational testing at this point?
MISIUKIEWICZ: I can say that, yes, even though [this patient] had the germline mutation negative, you can have a sporadic mutation, so it’s always worth testing the patient and testing the tumor. That would be my only reason, and I understand that maybe sometimes you may have some problems acquiring the tissue, but I think it’s necessary. And would you hold any mutational testing at this point? I mean, we ordered the whole sequencing in my institution. We used Foundation One and similar testing, and I think those are quite commonly available testing that we can use. And probably those are the ones that we can order for this patient.
When you say there’s concern about having tissue to send, is liquid biopsy then effective for this tumor type?
MISIUKIEWICZ: At least, there is no evidence for this, but this is what I would say. The treatment for thyroid cancer is thyroidectomy. I cannot imagine a situation unless the thyroidectomy was done 20 or 10 years ago—then it’s going to be hard to acquire the tissue. And so most of the time, I have access to the post-surgical thyroidectomy, and if you have the mutation the patient will have it in the specimen. So, there is no evidence, at least as of this moment, about liquid biopsy for those patients, for medullary thyroid cancer.
KOTIAH: [The patient] now has liver metastases and is a lot more symptomatic, but is it common to wait 6 months to rescan—because he went from asymptomatic lymph nodes to a lot more burden of disease and a lot more symptomatic 6 months later?
MISIUKIEWICZ: Most of the patients with medullary thyroid cancers are going to have this mutation, about 60% to 90%. You may see papillary thyroid cancer, and I have personally seen a patient with this mutation—it’s going to be a fusion mutation, and it only happens in 10% to 20% of patients. So, it’s a very common mutation that we see in medullary thyroid cancer, but it’s not very common in papillary thyroid cancer. And obviously, there are some other mutations. There is going to be difference in efficacy for medullary thyroid cancer, your point mutations, and in papillary thyroid cancer with an excel-lent response rate.
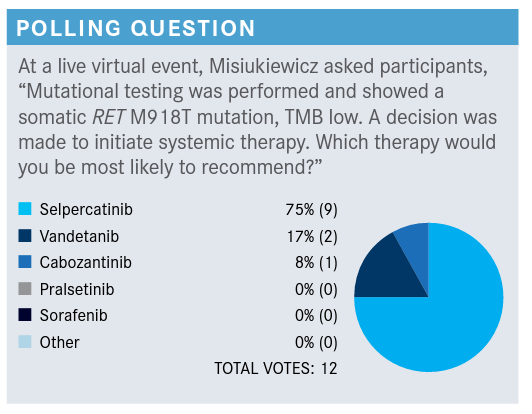
Please discuss these poll results.
MISIUKIEWICZ: There is a study of ponatinib [Iclusig]. In this study, the requirement was that a patient who had medullary thyroid cancer didn’t have to show evidence of progression.1 They’re taking patients that have a very indolent disease, and they put them on the study, and patients were randomized to ponatinib and placebo, and on the placebo arm, the PFS [progression-free survival] was 18 months. So it’s quite a long time. What I’m trying to say is that this disease can be indolent...can be slow-going, so there are some situations that you can consider active surveillance rather than the active treatments.
I have a tendency, for those patients, to initiate the therapy quite early, but there are 2 kinds of scenarios that you can see: You can have a patient that, let’s say, has a 2-cm mass, and you’re repeating the scans, and the mass is growing by 2 mm, and the patient is asymptomatic, but this is evidence of the progression and radiographic progression, so you can consider the treatment for this patient. On the other hand, you can have a patient who is symptomatic, but there is no evidence of radiographic progression. Which patient would you treat?
I would say, you have to consider treatment for both. Obviously, you’d have to kind of put the clinical scenario into the picture. For this particular patient presented here, I would initiate a treatment much earlier, unless there is something in the history or patient has some concern about the adverse events or there are some kind of other reasons not to initiate therapy. However, I would advocate for earlier rather than later to start the treatment, especially if the patient has liver metastases.

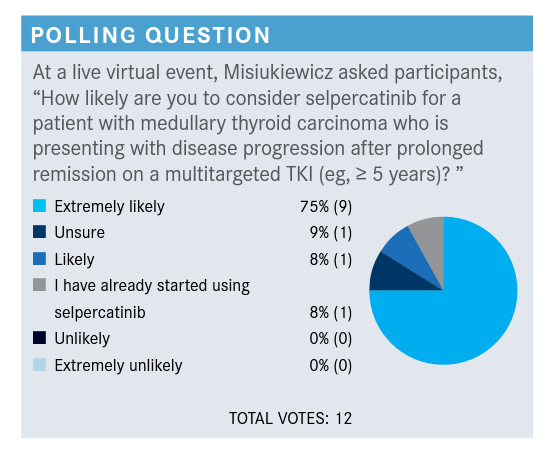
What is your reaction to these data, and how does this impact potential treatment decisions?
BHANDARI: [There was a] 100% response rate in not [previously] treated [RET fusion—positive] patients. That’s remarkable and a very good response, even if it’s some-times 60%, 70%, or 80% response. It’s very good.
MISIUKIEWICZ: Correct. I had 1 patient that I was planning, because of the kidney promulgation, to give them pralsetinib. So I play to the insurance, the response from insurance [at the time] was, “We won't approve this drug, because it’s not FDA-approved,” and I had to send them the screenshot from the FDA website. I said, "You are outdated. This drug has been approved." So I always kind of encourage you to do your due diligence, because you can be faster as a physician before some of these insurance companies. Because of the cost, they’re a little bit hesitant to [approve payment for this drug]but yes, the data are amazing. Obviously, it’s a short followup, so we have to keep our eyes open still.
What is the design of the phase 1/2 ARROW (NCT02412878) trial that looked at pralsetinib (Gavreto) for patients with RET-mutant medullary thyroid cancer?
MISIUKIEWICZ: [Patients with] advanced solid tumors, if they...had RET mutations, patients with prior cabozantinib and vandetanib [n = 67], RET-mutated patients with no prior systemic treatment [n = 42], and then we have RET-mutated patients with prior systemic treatment other than cabozantinib [Cabometyx] and vandetanib [Caprelsa], and [that was] 10 patients.3
[The median age of patients on the study] was around 60 years old, predominantly male, predominantly Caucasians, and with good performance status. The involvement is 7% vs 14%, depending on [if the patient is] cabozantinib-naive, or vandetanib-naïve, or was on one of the previous treatments.
Obviously, there are quite a bit of treatments that the patient received in the past, and some of them they even received anti–PD-1 or other kinase inhibitors.
What were the efficacy and safety outcomes of this study?
MISIUKIEWICZ: The overall response rate was 60% (95% CI, 46%-73%) [in the RET-mutant arm] and 66% (95% CI, 46%-82%) [in the cabozantinib-naive and vandetanibnaive arm]. [Comparing these data to selpercatinib at 69% and 72%, respectively,] complete responses here were 1.8% and 10%, respectively. Partial response is 58% vs 55%, and median duration of the response was not registered. Duration of the response of more than 6 months was 79% and 84%. So again, [a] very powerful drug.
In terms of the [adverse events (AEs)], one of the common AEs is hypertension. There is also some musculoskeletal pain, constipation, dry mouth, pneumonitis, [and] nausea, but the hypertension was the most common. There was some neutropenia, [and] there was some tumor lysis with pralsetinib, so this is something that we have to take [into] consideration. As we remember from the lung cancer, there is some pneumonitis that has been registered [with this treatment].
Ultimately, the FDA approved pralsetinib for RET-altered thyroid cancer, and it happened in December.⁴ There is some tumor lysis that has been reported with pralsetinib, so you have to watch those patients carefully, especially if somebody has a huge tumor, a huge tumor burden, and renal dysfunction. So this was one of the risk factors, so you have to hydrate them properly.
What is your reaction to these data for pralsetinib, and what stands out to you?
MISIUKIEWICZ: I would say that if you’re going to [look at AEs], the tumor lysis can happen regardless of the drug. It can happen with pralsetinib and selpercatinib, so I don’t think that we should think that one of them offers the lower risk. I think it’s more about the tumor burden rather than the drug itself.
There are some differences in efficacy, but obviously, [they’re] different populations of patients. I think the differences are that one option is given once a day [and] the other one is given twice a day, so you may have some preference.
The selpercatinib has some promulgations concern… so maybe you would be more inclined to use pralsetinib instead of selpercatinib. If you have a patient with a high risk of pneumonitis, then selpercatinib is going to be a better choice. There are some neutropenia concerns with pralsetinib, so considering COVID-19, maybe selpercatinib is going to be a better choice.
But those are very, very minor differences. I think they’re both pretty good drugs. And [I’m hoping that] having more drugs on the market, maybe the price will go down.
What are your thoughts on both treatments?
MISIUKIEWICZ: I have personally used both drugs, and they’re quite effective. I like pralsetinib and selpercatinib. There is always a question about sequencing—which drug you would use first. I would use the least toxic and most efficacious, so I would prefer to use the pralsetinib and selpercatinib as a first-line treatment, even though the response rate was quite similar with patients who were treated with [tyrosine kinase inhibitors] before. But that would be my preference.
KORYTKO: I don’t know how many thyroid cancers I’ll see in my career, but since you’re using the paradigm for aromatase inhibitors, if you have an excellent response to one of the last 2 drugs, can you use them in sequence? If you’ve had a good response to one, maybe there’s [an adverse] effect issue, but if you lose response to one, can you use them in sequence? Any experience with that?
MISIUKIEWICZ: No, I don’t have any experience [with that]. It is an excellent question, you know, I personally wouldn’t use them in sequence. I would eventually consider changing them if, let’s say, the patient develops promulgations then I wouldn’t have any problems to change to the other drug, obviously, as long as the insurance is going to approve it.... Unless, there is a possibility that you may have a unique patient that they have the mutation that obviously was kind of reported on the study. The most common is 918, so I would look for those, but as far as I know, I don’t think that there is a difference, and I wouldn’t be in favor of sequencing them.
MISIUKIEWICZ: OK, so as we know in melanoma, we have a different paradigm. When we have PD-1, and we have the immunotherapy for BRAF-mutated patients, and we have the BRAF inhibitors, historically, we used to favor BRAF inhibitors over immunotherapies. And now we cannot. We reversed it, and we cannot give immunotherapy prior to giving BRAF inhibitors. So what do you think about the patient who has presented today, having this in mind? Would you be in favor of using PD-1, or would you be in favor of using the RET inhibitor?
HARPER: I would be in favor of using the RET inhibitor. I think the responses are extremely robust. I look forward to getting more experience with them. I have one patient on selpercatinib now. Her response has been quite robust. She’s tolerating the treatment well. She likes taking it at home [and] not having a lot of office visits for treatment. [She] comes in once a month for blood work and monitoring [and] gets an [ECG] done. It’s pretty clean and gratifying. I think it’s nice to have the checkpoint inhibitor as a backup.
References:
1. National Library of Medicine (U.S.). (2019, Feburary-2021, May). Ponatinib in Advanced or Metastatic Medullary Thyroid Cancer. Identifier NCT03838692. https://clinicaltrials.gov/ct2/show/NCT03838692
2. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825-835. doi:10.1056/ NEJMoa2005651
3. Hu M, Subbiah V, Wirth LJ, et al. Results from the registrational phase I/II ARROW trial of pralsetinib (BLU-667) in patients (pts) with advanced RET mutationpositive medullary thyroid cancer (RET+ MTC). Ann Oncol. 2020;31(suppl 4):S1084. doi:10.1016/j.annonc.2020.08.1401

Anticipating Novel Options for the RAI-Refractory DTC Armamentarium
May 15th 2023In season 4, episode 6 of Targeted Talks, Warren Swegal, MD, takes a multidisciplinary look at the RAI-refractory differentiated thyroid cancer treatment landscape, including the research behind 2 promising systemic therapy options.
Listen
Conservative Management Is on the Rise in Intermediate-Risk Prostate Cancer
January 17th 2025In an interview with Peers & Perspectives in Oncology, Michael S. Leapman, MD, MHS, discusses the significance of a 10-year rise in active surveillance and watchful waiting in patients with intermediate-risk prostate cancer.
Read More
What Is Dark Zone Lymphoma, and Is It Clinically Relevant?
January 16th 2025Dark zone lymphoma includes aggressive B-cell lymphomas with shared molecular features. While some respond to escalated treatment, others remain resistant, highlighting the need for targeted approaches to improve outcomes.
Read More
Controversy Swirls Around the Use of CDK4/6 Inhibitors as Adjuvant Breast Cancer Therapy
January 15th 2025CDK4/6 inhibitors like abemaciclib and ribociclib improve invasive disease-free survival in breast cancer trials, but controversy surrounds study designs, bias, and cost-effectiveness, raising critical questions about their clinical benefit.
Read More