PARP Inhibitors an Option for Metastatic Castration-Resistant Prostate Cancer
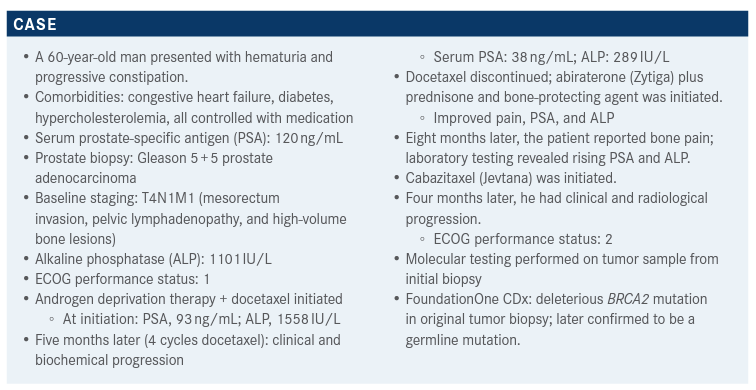
During a Targeted Oncology Case-Based Roundtable event, Tian Zhang, MD, MHS, an assistant professor of Medicine at Duke Cancer Institute, discusses the case of a 60-year-old patients with metastatic castration-resistant prostate cancer.
During a Targeted Oncology Case-Based Roundtable event, Tian Zhang, MD, MHS, an assistant professor of Medicine at Duke Cancer Institute, discusses the case of a 60-year-old patients with metastatic castration-resistant prostate cancer.
Targeted OncologyTM: What are your thoughts and practice regarding genetic counseling and germline testing for such patients, in light of the recommendations of the National Comprehensive Care Network (NCCN) guidelines?
ZHANG: The NCCN discusses both germline [and] somatic testing.1 I [think] that germline [testing] really is helpful for patients who have a strong family history or high-risk metastatic disease. I will often ask our fellows to really drill into family histories of cancer; if [the patients are] higher risk, we’ll talk about germline testing early. Prostate cancers can certainly show up in Lynch syndrome and also [with] any of the homologous recombination [repair] [HRR] gene mutations. I think [testing is] important for risk stratification and counseling for their children. We [explain it to patients this way:] most of the time, we don’t find anything, [but] in case we do, we’ll send you to the genetic counselors to discuss risk for your family members and children. I think risk for [patients’] family members is something important to think about.
I do think the somatic tumor test will [inform] treatment and the actionability of a PARP inhibitor [such as olaparib (Lynparza)2 or rucaparib (Rubraca)].3 The NCCN recommends somatic testing for homologous recombination deficiency if the patient has metastatic disease, and [suggests that such testing also be considered in the case of] regional disease.1
[According to the NCCN, somatic testing for] microsatellite instability [should also be] considered for regional or for early hormone-sensitive disease, and [such test-ing] is recommended for metastatic castration-resistant disease. If you have a microsatellite-unstable patient and you [suspect] Lynch syndrome, certainly there is an indication for immunotherapy with pembrolizumab [Keytruda] in that setting. Multigene molecular testing should be considered for anybody with localized prostate cancer and a life expectancy greater than 10 years. Finally, there is Decipher molecular testing, which many of our urologists do, they will send after prostatectomy the original prostatectomy specimens.1
What data support the use of olaparib in patients like the one described? The phase 3 PROfound study [NCT02987543] [looked at] metastatic castration-resistant prostate cancer [mCRPC] [in patients who had] progression on [a prior new hormonal agent, such as] abiraterone or enzalutamide [Xtandi]. They enrolled more than 4000 patients and looked for [alterations in] 15 prespecified HRR genes. [Some familiar ones are] BRCA1, BRCA2, and ATM. Some of the less familiar ones are things like CDK12, CHEK1, and CHEK2, and also the RAD genes. Of the [over] 2700 samples that were [examined], 778 (28%) had at least 1 mutation [in the prespecified genes]. Of these 778 patients, 387 (50%) ultimately met all of the eligibility criteria and went on to the trial.4
Cohort A were patients who had BRCA1, BRCA2, or ATM mutations, and cohort B were patients with other HRR defects. Patients [in each cohort] were randomly assigned [to receive] either olaparib or physician’s choice [of abiraterone or enzalutamide]. This trial allowed patients [who had previously received chemotherapy and those who had not]. The primary end point was radiographic progression-free survival [PFS] in cohort A; a key secondary end point was radiographic PFS in cohorts A and B [together]. [Other secondary end points were] the confirmed radiographic [objective] response rate [ORR], time to pain progression, and overall survival [OS], all in cohort A.4
Regarding the primary endpoint (radiographic PFS in cohort A), the hazard ratio [HR] was 0.34 [95% CI, 0.250.47; P < .001] favoring olaparib, with a median PFS of 7.4 months vs 3.6 months with the control.4
If we combine cohort A and cohort B (to include all of the other [altered HRR] genes), the HR goes up but [still] favors olaparib [HR, 0.49; 95% CI, 0.38-0.63; P < .001]. The median PFS here was 5.8 months vs 3.5 months. [Analysis was performed on] prespecified subgroups, [including groups defined in terms of] previous taxane use, [yes or no]; measurable disease [at baseline, yes or no]; [presence of] bone-only metastases, visceral metastases, or other metastases; and geographic region [Asia, Europe, or North and South America]. These seemed to all favor olaparib, although the Asia subgroup leaned a little toward an HR of 1.00 [HR, 0.67; 95% CI, 0.44-1.04] [and no prior taxane use crossed 1.00 (HR, 1.03; 95% CI, 0.57-1.92)].4
What did this study reveal about the effect of specific gene alterations on PFS? Certainly BRCA2 [mutation] favored olaparib [HR, 0.21; 95% CI, 0.13-0.32]. BRCA1 has a very large confidence interval, probably because of few events [HR, 0.41; 95% CI, 0.13-1.39]. ATM, CDK12, and CHEK2 all [had CIs that overlapped] an HR of 1.00. PPP2R2A actually favored the control [HR, 6.61; 95% CI, 1.41-46.41], and RAD54L had a very large confidence interval [HR, 0.33; 95% CI, 0.05-2.54]. These subgroups [also] probably had very few events.4
What effect did olaparib have on OS in the different cohorts and subgroups?
Median OS was 19.1 months [95% CI, 17.4-21.4] in cohort A and 14.7 months [95% CI, 11.9-18.8] in cohort B [the control cohort]. The HR favored olaparib [HR, 0.69; 95% CI, 0.50-0.97; P = .02]. Two-thirds of these patients crossed over and received olaparib after the control was finished, and [for this group] the HR was 0.42 [95% CI, 0.19-0.91]. OS, [when analyzed] by subgroup in cohort A, favored olaparib in all subgroups: prior chemotherapy, measurable disease [at baseline], metastasis [at baseline], ECOG performance status, age, geographic region, race, and baseline PSA.5
OS [was also analyzed] for the overall population (cohort A plus cohort B). Here, the [difference in] median OS is a little smaller, 17.3 months [95% CI, 15.5-18.6] for olaparib, 14.0 months [95% CI, 11.5-17.1] for control, with an HR of 0.79 [95% CI, 0.61-1.03]. In the two-thirds of patients who crossed over, there was a [slightly greater] difference [in median OS], with an HR of 0.55 [95% CI, 0.29-1.06].5 In cohort A, the confirmed ORR was 33.3% among patients treated with olaparib and 2.3% among patients treated with physician’s choice [of abiraterone or enzalutamide]. The odds ratio [OR] here was quite high [OR, 20.86; 95% CI, 4.18-379.18; P < .0001] favoring olaparib.6
Can you describe the data on adverse events [AEs] following treatment with olaparib vs the control? Most patients did have an AE [95.3% in the olaparib group, 87.7% in the control group]. [In these same groups], AEs of grade 3 or higher occurred in 50.8% vs 37.7% of patients in these respective groups. Dose reduction due to an AE occurred in 22.3% of olaparib patients compared to 3.8% of control patients, and discontinuation [of treatment] occurred in 16.4% vs 8.5%, respectively. The rate of death due to AEs was comparable between the 2 cohorts [3.9% and 3.8%], and only 1 AE-related death in each cohort was thought [to be] related to study treatment.
[Patients had] significant anemia attributable to olaparib, something we think about a lot when patients are on treatment. Nausea and fatigue can be extreme in some cases. Decreased appetite, diarrhea, and vomiting are all common as well. The pulmonary embolism rate was 4.3% with olaparib vs 0.8% with physician’s choice; none of these were fatal. There were no reports of myelodysplastic syndrome or acute myeloid leukemia, although certainly we have known [through] anecdotal experience that [they] can be associated with PARP inhibitors [such as] olaparib.5,6
Acute kidney injury [AKI] was not mentioned. Have you seen any AKI in your practice?
I have not seen AKI [resulting] from tubular injury or anything like that. I know of an anecdotal report of asymptomatic AKI in a patient with prostate cancer. After the patient was taken off the drug and normalized, the patient resumed treatment on a lower dose and did fine.
What can you tell us about the current clinical application of olaparib?
In May of last year, we had the approval of olaparib for use in HRR gene–mutated mCRPC.7 Then in November, the FDA approved the blood-based FoundationOne Liquid CDx test to be used as the companion diagnostic [to olaparib]8 and that was the test that was used in PROfound4 as well.
Many [people] use the Guardant test as well; in my practice, we have not had trouble getting olaparib when we found [mutations with] the Guardant test.
What other therapeutics have been recently tested for use in patients with mCRPC and HRR gene alterations?
TRITON2 [NCT02952534] was a phase 2 study of rucaparib in patients with mCRPC with either somatic or germline mutation in an HRR gene, disease progression on an androgen receptor–targeted therapy, and 1 prior taxanebased chemotherapy. A good performance status was necessary to go on the study, and no prior [treatment with] PARP [inhibitors, mitoxantrone, cyclophosphamide, or platinum-based chemotherapy] was allowed. All patients were treated with rucaparib, 600 mg twice a day, and then assessed for tumor progression. Patients were stopped if they had radiographic progression or AEs. The primary end points were ORR and PSA response rates.9
They screened 1781 patients; 209 were enrolled, and out of these, 115 had deleterious BRCA mutations; at data cutoff, about 25% of the patients remained on treatment, and the median follow-up was 17.1 months [range, 7.6-31.5]. Of all the patients with BRCA mutations, 61.7% had somatic mutations and 38.3% had germline mutations. Many of these patients had biallelic [alteration zygosity] [31.3%], although the majority had unknown zygosity status [60.9%].
What was the patients’ response to rucaparib treatment in this study?
The radiographic responses for patients within the independent radiology review [IRR]-evaluable populations were as follows. The ORR in terms of the sum of target lesions (change from baseline) was 43.5% [95% CI, 31.0%-56.7%]. Some of these patients actually obtained a complete response, 6.2% in the investigator-evaluable population and 11.3% [in the IRR population]. The response rate in terms of PSA change from baseline in the overall efficacy population was 54.8% [95% CI, 45.2%-64.1%]. Only [about] 9% of patients [both in the investigator-evaluable population and in the IRR-evaluable population] had primary progressive disease, which is quite encouraging. For patients with BRCA1 and BRCA2 mutations, the median radiographic PFS by IRR was 9.0 months [95% CI, 8.3-13.5] and median OS was not mature at that time, [though] the estimated 12-month OS rate was 73.0% [95% CI, 62.9%80.7%]. Median time to PSA progression [in the overall efficacy population] was 6.5 months [95% CI, 5.9-7.8].
What subgroup analysis, if any, was performed? Seventy-eight patients were enrolled [in TRITON2] with a non-BRCA [DNA-damage response] gene mutation. [There were separate] cohorts with mutated ATM, CDK12, and CHEK12, and [a final cohort labeled] “other.” These were quite small cohorts; [those with] CDK12, CHEK12, and “other” [mutations] were particularly small. Very few of these patients, if any, had a complete response [7.1% for “other,” 0% for the other cohorts]. Partial responses occurred in 10.5% of patients with mutated ATM, 11.1% of those with mutated CHEK2, 21.4% in patients “other” mutations, and in none of the patients with mutated CDK12. In general, the data for objective responses looked better for patients with BRCA1 and BRCA2 mutations [in the data reviewed above] than for patients with non-BRCA mutations.
What were the AE data for rucaparib?
The median treatment duration for patients assessed for AEs was 3.7 months [range, 0.5-12.9], and for patients who had BRCA1 and BRCA2 mutations, the duration was 4.4 months [range, 0.50-12.0]. In the overall safety population, most patients [95.3%] had at least 1 AE [of any grade], and 52.9% had at least 1 AE of grade 3 or higher. There were treatment interruptions [due to AEs] in 48.2% of patients, dose reductions [due to AEs] in 29.4% of patients, and treatment discontinuation due to AEs in 5.9% of patients. [Finally, there was] 1 AE-associated death.11
The AEs of any grade that were the most common [greater than or equal to 10%] in [the overall safety population] included asthenia, nausea, and anemia, [which was the most common AE among those of grade 3 or higher]. Monitoring [liver function] is important; [the percentage for elevated alanine or aspartate aminotransferase] of grade 3 or higher was 4.7%.
What can you tell us about the current clinical application of rucaparib?
Based on the [data], the FDA granted accelerated approval to rucaparib for BRCA-mutated mCRPC12 and also approved FoundationOne Liquid CDx test as the companion diagnostic.13
References:
1. NCCN. National Comprehensive Cancer Network. Prostate Cancer, version 2.2021. Accessed July 1, 2021. https://bit.ly/3yIXOBC
2. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123-134. doi:10.1056/NEJMoa0900212
3. Plummer R, Jones C, Middleton M, et al. Phase I study of the poly(ADPribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res. 2008;14(23):7917-7923). doi:10.1158/1078-0432.CCR-08-1223
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/ NEJMoa1911440
5. Hussain M, Mateo J, Fizazi K, et al; PROfound trial investigators. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383(24):2345-2357. doi:10.1056/NEJMoa2022485
6. Hussain M, Mateo J, Fizazi K, et al. PROfound: phase 3 study of olaparib versus enzalutamide or abiraterone for metastatic castration-resistant prostate cancer (mCRPC) with homologous recombination repair (HRR) gene alterations. Ann Oncol. 2019;30(suppl 5):v881-v882. doi:10.1093/annonc/mdz394.039
7. FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. News release. FDA. May 20, 2020. Accessed July 4, 2021. https://bit.ly/3hyVrMf
8. FDA approves liquid biopsy NGS companion diagnostic test for multiple cancers and biomarkers. News release. FDA. November 9, 2020. Accessed July 4, 2021. https://bit.ly/2VtIXg9
9. Abida W, Patnaik A, Campbell D, et al; TRITON2 investigators. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020;38(32):3763-3772. doi:10.1200/JCO.20.01035
10. Abida W, Campbell D, Patnaik A, et al. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the phase II TRITON2 study. Clin Cancer Res. 2020;26(11):2487-2496. doi:10.1158/1078-0432.CCR-20-0394
11. Abida W, Bryce AH, Vogelzang NJ, et al. Preliminary results from TRITON2: a phase 2 study of rucaparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) associated with homologous recombination repair (HRR) gene alterations. Paper presented at: European Society of Medical Oncology; October 19-23, 2018; virtual. Accessed July 4, 2021. https://bit. ly/3hyKVo3
12. FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer. FDA. May 15, 2020. Accessed July 4, 2021. https://bit.ly/3ee6EzK
13. FDA approves liquid biopsy next-generation sequencing companion diagnostic test. News release. FDA. November 15, 2020. Accessed July 4, 2021. https://bit.ly/3k5M7kA
