Roundtable Discussions: Participants Compare Avelumab Maintenance With Other Bladder Cancer Therapies
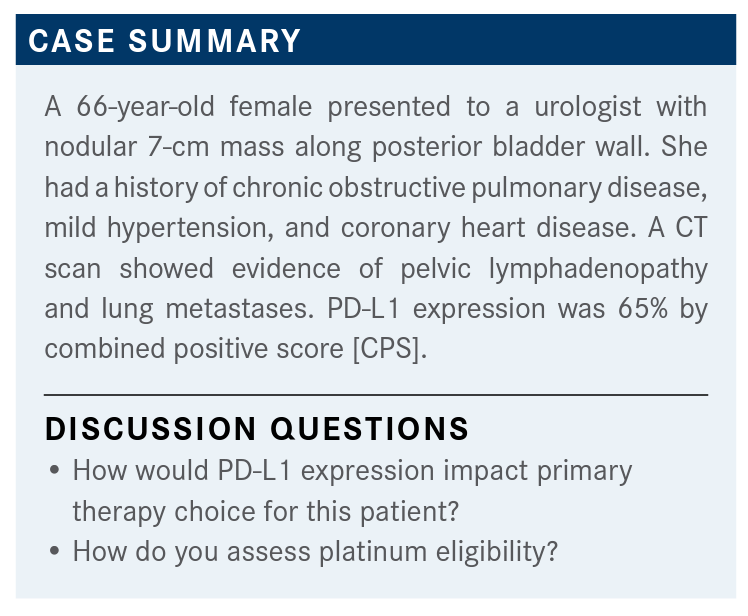
Jorge A. Garcia, MD, FACP, discussed that case of a 66-year-old female presented to a urologist with nodular 7-cm mass along posterior bladder wall and was later diagnosed with bladder cancer. He was joined by Neeraj Mahajan, MD, Anish B Parikh, MD, and 8 other physicians during a Targeted Oncology Case-Based Roundtable event.
Jorge A. Garcia, MD, FACP

Jorge A. Garcia, MD, FACP, division chief of Solid Tumor Oncology and the George & Edith Richman Distinguished scientist chair at the University Hospitals Seidman Cancer Center, discussed that case of a 66-year-old female presented to a urologist with nodular 7-cm mass along posterior bladder wall and was later diagnosed with bladder cancer. He was joined by Neeraj Mahajan, MD, Anish B Parikh, MD, and 8 other physicians during a Targeted Oncology Case-Based Roundtable event.
GARCIA: So, there are 2 agents that we do PD-L1 staining for. One is atezolizumab [Tecentriq] and the second is pembrolizumab [Keytruda].1,2 In both cases, we use different antibodies, and the definition for overexpression is graded differently because the cells that we test for are different for the Ventana assay for atezolizumab, and also the CPS.3
How do we think of this patient in terms of assessing her eligibility for treatment? None of her comorbidities are a concern to me. We didn’t cover her functional capacity, but let’s assume that her ECOG [performance status] or KPS [Karnofsky performance status] was okay.
We didn’t talk about neuropathy. She didn’t have any evidence of hearing loss. She didn’t have any history of heart failure. But the reality of it is, her GFR [glomerular filtration rate] was 50, which puts a lot of pressure on our ability to actually give someone cisplatin-based chemotherapy...not carboplatin, but cisplatin, for sure.
How do we assess platinum eligibility? I would argue, the question is, is it platinum or cisplatin eligibility? If you look at patients who are chemotherapy unfit—which is platinum, carboplatin, or cisplatin unfit— you look at the consensus definitions that have been put forward by many [investigators] in the past, including Matt Galsky, MD, and Gupta et al.4,5 [Galsky surveyed medical oncologists internationally and Gupta in the United States to try to] define a consensus guideline as to how we think of these patients and...who is an unfit patient for chemotherapy.
[Ineligibility for cisplatin or carboplatin from the Bladder Cancer Working Group consensus definition is based on having 1 of the following]: ECOG PS greater than or equal to 3, a creatinine clearance [CrCl] less than 30, grade 3 [or higher] peripheral neuropathy, or heart failure [by New York Heart Association (NYHA)] class III. There is no doubt that if you have [a patient] like that, it’s very unlikely that you’re going to be able to put that patient through chemotherapy. So that patient could be a great candidate for immunotherapy mono-therapy with either atezolizumab or pembrolizumab, which the FDA has approved.1,2
Over the past month or so—without me having vested interest in the FDA or in the companies—the FDA was reviewing whether atezolizumab and pembrolizumab should remain in the market in that space [as] subsequent trials have not shown survival improvement.6 [There is the] exception, obviously, of the second-line pembrolizumab data.
Now we [also have] the Galsky criteria, which again is a consensus definition by virtue of a survey of international GU [genitourinary] oncologists.4 [These criteria only pertain to cisplatin, not carboplatin.] [Having 1] or more of the following items would define a patient as cisplatin unfit: an ECOG PS of 2 or greater or a KPS of 60% to 70%, creatinine clearance less than 60, grade 2 audiometric hearing loss, grade 2 peripheral neuropathy, or NYHA class III heart failure. So when you compare [the ineligibility criteria], the biggest difference here is the degree of neuropathy and also the GFR for that patient, along with the functional capacity.

GARCIA: [For] this patient, would you use the GFR of 50 as the sole reason to start immunotherapy? Or would you feel comfortable giving this patient carboplatin- or cisplatin-based chemotherapy?
MAHAJAN: This patient, I think, is platinum eligible. And I think we still have long-term data and the most extensive data [with platinum]. So, I’ll still try to use GC [gemcitabine plus cisplatin] as my preferred regimen.
GARCIA: That means that you’re not bothered by her GFR of 50. And the reason why I’m [focusing] on this is because this [is the] controversy among all of us in the GU field. Is it [a GFR of] 50, or is it 55? What if it is 49? Could you hydrate the patient, and so forth? I think it’s fair to say that some of my colleagues around the country will [have patients with] a GFR of 50 and feel comfortable giving these patients cisplatin. I think under 50, most of us would not.
My cutoff is 55. But I also look at comorbidities to make that decision. For the most part, I would not give cisplatin-based chemotherapy to someone with a GFR under 50. Between 50 to 55, we’ll have to look at the reason why their GFR is between 50 to 55. Over 60, I agree, everybody [is eligible for platinum-based chemotherapy].
PARIKH: I think that 50 to [60] borderline region is always controversial. I think it’s important for everyone to consider in those situations...[whether] there is a reversible cause. With these primary bladder tumors, oftentimes there is some degree of hydronephrosis, either unilateral or bilateral. And if any amount of renal function can be salvaged or recovered by decompressing the urinary system, I think that’s important to do as quickly as you can. [Cisplatin is] clearly the best drug that we have, chemotherapy-wise, for bladder cancer. It would be a shame to not try to get a patient that best treatment, if that’s the only thing standing in the way and if it’s something that can be reversed.
GARCIA: Great point, Anish. [You] mentioned the gray areas with respect to cisplatin ineligibility. When I was in the clinic, we published data looking at the impact of cisplatin-based chemotherapy for [patients] with impure GFRs; after they got decompressed, [their GFR] improved. And the likelihood of renal dysfunction was greater for those patients compared with historical controls. Although you can mathematically correct the GFR, dependent on the timing, and that’s why Anish mentioned [that] you have to rapidly [address] these things. If a patient is compressed for 1 week, or 2 weeks, or 3 weeks, it’s different than someone who’s been running with GFRs in the 40s to 50s with obstructive disease for 1 or 2 months.
What other factors do you pay attention to when you [consider ineligibility criteria for platinum therapy]?
SABAGH: I look at performance status as a main factor.
GARCIA: Great. How about if I were to tell you that the patient’s functional capacity is completely dependent on her disease? Basically, she’s just impaired by virtue of the cancer growing. Would that change your mind [about] her PS and [affect your] choices in treatment?
SABAGH: I usually ask the patient how their functional status [was] a month or 2 before that. I try to find a baseline and see how much that has to do with the cancer, and if you correct it, you improve that. Many times, especially with elderly patients, when they have [an ECOG PS] of 2 or 1.5, they generally don’t recover. If you have some patients who are [in their] late 80s with borderline PS, I would worry about the cisplatin.
MAHAJAN: I [consider] the neuropathy, heart failure, baseline cardiac function, and hearing deficit, along with performance status and renal function....
GARCIA: It’s funny you say that because most of [our patients with] bladder cancer [have a] median age [in] the high 60s or 70s. And if you pay attention, a lot of [patients] have hearing devices. Often I don’t do audiometries....I think if I wanted to give you cisplatin I’ll do it, but I could use that as an excuse not to do it. I think most of us in practice will do it.
How about neuropathy? If you have [a patient] with significant diabetes and they have a history of neuropathy, would that be a reason for you not to put that patient on cisplatin?

KUMAR: Again, I will consider other factors, also, not just neuropathy. If a patient has mild neuropathy and other factors, probably I’m not going to use it. If somebody has grade 3 or more neuropathy, probably I will stay away from it.
GARCIA: We’re talking about cisplatin, but none of us are using cisplatin alone. Who in the group uses ddMVAC?
CHOWDHARY: I have used it in 2 or 3 patients. Usually, middle-aged or younger patients who are functionally doing very well. I’ve used ddMVAC in the stage IV setting, just based on my experience using it in the neoadjuvant setting. I know that the data may say otherwise, but I still feel it’s a better regimen than GC alone. Having said that, I think 75% of my patients still go on GC, followed by sequential immunotherapy. But I’ve [had] a lot of good experience with ddMVAC.
GARCIA: ...When you say you think that ddMVAC is better than GC, what do you think is better? Is it responses? Is it PFS [progression-free survival], is it survival?
CHOWDHARY: Maybe not survival, but the response rates are better. Again, everything we’re doing here is for a palliative benefit. They may have a large bladder mass, they may have other tumor burden, so I think any higher response rate will translate into somewhat better palliative rate. And then, again, I think it’s really response rates and even the rates of CR [complete response] in the neoadjuvant setting that we find. I always think about ddMVAC whenever I’m seeing a relatively healthy patient.
GARCIA: I agree. I have used it, I can count [the times on] 2 hands, but it usually tends to be in a super-young, maybe 40-year-old patient. My [experience with it] is just limited, so it’s just hard for me to comment. But if I were to use cisplatin for this patient with a GFR of 50, I may just say, “Well, I’d love to use the split-fashion cisplatin, it minimizes renal dysfunction.”
What is your favorite schema of GC if you were to use it? Do you use the European version, the 1, 8, 15, break on 21, and move it out to 28-day cycle? Or do you do the 21-day cycle, or a split?
KNAPP: When I used it, I’ve done the 21-day cycle. I’m not opposed to doing it a different way, that’s just the standard that we have; the regimen we have in our system is the 21-day cycle.
NEMUNAITIS: In that young patient, where you might use ddMVAC, wouldn’t you use paclitaxel plus GC instead of MVAC in that patient, where you need a response, or is MVAC usually your go-to regimen?
GARCIA: That’s a great question. The only way that I can answer you that is that we did an intergroup phase 3 trial [NCT00022191] looking at GC against GC plus paclitaxel, and there were no outcome differences.7 So, triplet therapy may give you a better response rate, but there was no PFS and survival improvement, and that’s why the triplet regimen was abandoned. The reason why that trial was put forward is because paclitaxel, at the time, used to be a second-line choice in [the United States], but it really doesn’t add anything. I’m not sure that I think ddMVAC is a better regimen than GC, I just think that you may get perhaps a faster response than a better response, but the question is perhaps toxicity.... So, if I were to do that, I would probably do ddMVAC.
PARIKH: It’s very similar for me, I can probably count on just 1 hand the number of times I’ve done ddMVAC when I was not a fellow. Mainly because I just think those patients are hard to come by for bladder [cancer]. The patients tend to be older and they tend to be very frail and have a lot of comorbidities. But I have done it, [and it] has worked well in the cases where I’ve done it. I generally use the standard 3-week GC. I very frequently split. And maybe half the time, I’m splitting cisplatin day 1, day 8. And then, [I use] the ddMVAC for those rare patients I think can tolerate it.
But the 1 case where I tried to use paclitaxel plus GC, it went very poorly and that turned me off to it. I think that I’d probably just rely on ddMVAC if I felt [as though] I needed something more than the GC in terms of response rate.
CHOWDHARY: The reason I brought up ddMVAC was that obviously [for] the vast majority you’re considering more GC; we do use the split-dose gemcitabine on quite a fair number of patients. There [are] no data at all that [show that] using split course affects response rates in any way, are there? I’m not aware of any.
GARCIA: There [are] no data. I agree with you, it’s that anecdotal practice behavior, which I love. I think my own anecdotal experience is that it just really has a little bit less renal impact and less dumping syndrome with potassium and electrolytes. Maybe it’s also because patients are getting [intravenous] fluids twice instead of only on day 1.
But you’re right, there are no data. Also, we don’t have data on GC against ddMVAC. The von der Maase paper was GC vs MVAC, and the purpose of that trial was to demonstrate that GC was superior to MVAC.8
They failed the primary end point, although mathematically for us it became the standard of care just by virtue of tolerability, and the lack of mathematical difference between response, PR [partial response], stable disease, PFS, and survival. But that trial was a negative trial.

GARCIA: Bo, what happens if a cisplatin-unfit patient comes to your office? What is the process that goes through your mind to select chemotherapy, or perhaps immunotherapy as monotherapy?
ZHAO: [For a] cisplatin-ineligible patient, chemotherapy still offers the highest response rate. For carboplatin-based [treatment], the response rate is around 50% to 60%, and that’s the best chance to achieve a response. If they could get chemotherapy, I would still prefer to give them chemotherapy.
[However, if they are] really not a chemotherapy candidate—[for example, they have] a performance status 3 or they have multiple comorbidities—I know if I give them 1 dose of chemotherapy, the next week they’re going to be in the hospital. [In that] specific setting, I would probably prefer single-agent immunotherapy.
GARCIA: Okay, that’s good. Shyamal, for [patients] who are chemotherapy fit but cisplatin unfit, how do you [decide] who gets carboplatin, who gets pembrolizumab, or even atezolizumab?
BASTOLA: I would think of carboplatin patients who are not eligible for cisplatin, specifically based on kidney function, and if they’re eligible otherwise. For those patients, if it’s not because their ECOG PS is 3, or if it’s not because they have severe other issues, then I would [think] about GC for them.
But, if they’re not eligible because of their poor PS and lots of medical comorbidities, then I think for those patients I would be thinking along the lines of immunotherapy, depending on their PD-L1 status.
GARCIA: You [brought up] the comment that I want to get to, and that is the use or utility of PD-L1 as a tool to define primary therapy for our advanced patients who are cisplatin unfit. So, Evan, do you use PD-L1 staining for someone who is cisplatin unfit but could get carboplatin?
LANG: I would like to check those biomarkers, if possible, even up front. I agree with other physicians that I would consider chemotherapy for patients [who are] cisplatin ineligible. I would consider GC, followed by immunotherapy. Particularly in this case, I’m not going to wait for the PD-L1 test results, but I would like to get [the results] just in case.
GARCIA: Those are great comments. Perhaps you can use my [following] statements as editorial comments.
My take on this is very simple and may be a bit different to some of you. When I see [a patient] who has cisplatin unfitness but could get carboplatin-based therapy, I believe there are sufficient data to use monotherapy with either atezolizumab or pembrolizumab instead of carboplatin.
[The reason for that is] there are 2 frontline trials that were conducted in a parallel fashion that [compared] pembrolizumab monotherapy, atezolizumab monotherapy, pembrolizumab plus chemotherapy, atezolizumab plus chemotherapy, and chemotherapy alone.9,10
[In these trials,] chemotherapy [with] cisplatin or carboplatin [was allowed]. Prior to [trial] entry, patients were required to have PD-L1 staining based upon the antibody and the CPI [checkpoint inhibitor] that they were using [in the study].
The important part of these data is that this is what the FDA [based its] change of the labels of pembrolizumab and atezolizumab on.11 There was an interim analysis for both trials, respectively, that demonstrated that [patients] who were getting monotherapy on those 2 separate trials were having detrimental outcomes.
When [the FDA] reviewed the data, it found out that the [patients] who were having detrimental outcomes were the [patients] who had [low expression] of PD-L1. The CPS score was less than 10%, and the IHC [immunohistochemistry] for atezolizumab was less than 5%. So that’s why the FDA changed the label for monotherapy in the frontline setting to allow use only on those patients who have overexpression of PD-L1.11
So, I start [by asking], can [the patient] get chemotherapy? If the answer is yes, [I] move to cisplatin. If the answer is no, the question is, can [the patient] get carboplatin? And if the answer is yes, then I will do a CPS score. [Because] I use pembrolizumab the most, I don’t use atezolizumab. If [the] CPS is less than 10%, I move [the patient] to carboplatin.
If the CPS score is greater than 10%, then I can have an open discussion [with the patient about] the balances between GC or monotherapy with pembrolizumab. Recognizing that when those data came out, we didn’t have the JAVELIN [NCT02603432] data with GC followed by immediate maintenance therapy.12,13
[This], to some extent, challenges the monotherapy concept. So, that’s how I think of those patients. It is actually quite interesting to hear [that] all of you check [PD-L1 expression], but it may not be what you use. And it sounds to me [that] the bulk of you will put the patient on chemotherapy, which is appropriate in my opinion.
JAVELIN also allowed best supportive care.12,13 Evan, [if I could ask you] to comment: If you [elected] not to give avelumab [Bavencio] maintenance, how would you follow or [treat] those patients after they discontinue chemotherapy?
LANG: Understanding that eventually this patient will progress, I would watch closely. I would probably see the patient at least every 3 months and get a scan every 3 months, initially.
GARCIA: Great. Mark, can [you] comment as to what your style of practice is? What are the key points of education that you talk to your patient [about] during that shared decision-making process?
Because [they] may come to you as an educated consumer and tell you, “I know that I don’t have to rush into therapy because these data only demonstrate that either you give me something or you don’t give me something. But I also know that you can put me on pembrolizumab if my disease grows a little bit. So why would I embark on avelumab now when I can get immunotherapy again down the road if, indeed, I need it?”
KNAPP: Well, certainly they can get immunotherapy down the road, but we know that not many [patients] are going on to second-line therapy. And the first-line data show significant survival benefit, which we’ve not seen with second-line [therapy], not nearly to the same degree. If they were feeling well after 4 to 6 cycles of therapy, I would definitely be encouraging them to take immunotherapy immediately rather than waiting, unless there is some other reason why they wanted to wait—if they want to do a life-changing trip with their spouse or something like that. But other than that, I’d be pushing them toward therapy.
GARCIA: Let me just ask the “elephant in the room” question: Who would feel comfortable giving pembrolizumab in this setting? The reason why I’m asking is because avelumab never had a lot of [the] market in bladder [cancer], and the agents that had really [gained] a lot of traction were atezolizumab and pembrolizumab. The bigger question then becomes, are these agents interchangeable...and why not pembrolizumab, which is the most common question that I get. My answer is [that] the maintenance data [are] only with avelumab, not with pembrolizumab; therefore, avelumab should be the agent.13,14
MAHAJAN: Some of us who are using these drugs from top to toe haven’t seen much difference in efficacy. [Avelumab] has the most infusion reactions, and [it is administered] every 2 weeks.15 Pembrolizumab is [administered] every 6 weeks, especially in maintenance.2 [This means] fewer visits, [fewer] infusion reactions. Data are data, but I have no doubt that it will show the same thing if it’s done in the same kind of clinical trial.
MAHAJAN: Just 1 quick question on this JAVELIN data. There is a great survival benefit, but the response rate was still, I think, 10% for intention to treat, and 14% for PD-L1 positive.12 So, very few patients are benefiting.
GARCIA: Remember...[ we] want to know for those with stable disease, and for those with PR, [can you] convert them into CR, or stable [disease] into PR? And that is the complexity of [these] data, as you pointed out, that mathematically the numbers are not impressive.12 But because the primary end point of the data was survival, that’s why responses become less important. Not irrelevant, but less important, because they were not primary end points for the study.
