Roundtable Discussion: Zakharia Considers Next-Line Therapy Sequencing for mCRPC
During a Targeted Oncology™ Case-Based Roundtable™ event, Yousef Zakharia, MD, discussed next-line therapy for a patient with metastatic castration-resistant prostate cancer who has received docetaxel and an androgen receptor–targeted therapy.
Yousef Zakharia, MD
Clinical Associate Professor
Director, Phase I Program
Coleader, Genitourinary Oncology Program
University of Iowa Holden Comprehensive Cancer Center
Iowa City, IA


CASE SUMMARY
A 75-year-old man presented with intermittent right hip pain. His physical examination was unremarkable except for a prostate nodule on rectal exam, and he was given an ECOG performance score of 1. His prostate-specific antigen (PSA) level was 16.2 ng/mL, and his transrectal ultrasound scan biopsy Gleason 5 + 4 grade group was 5. He had a negative bone and abdominal/pelvic CT scan and received a diagnosis of stage cT2N0M0 disease. External beam radiation therapy and androgen deprivation therapy (ADT) treatment were initiated and planned for 24 months. Six months after initiation of therapy, his PSA level was undetectable, so he was considered asymptomatic. He did not return for PSA follow-up.
Thirty-six months later, the patient scheduled an office visit and reported increasing hip pain and urinary frequency. His PSA level was now 29.4 ng/mL, and he had a testosterone level of 300 ng/dL. His bone scan showed evidence of 2 new lesions in the right hip, and an abdominal/pelvic CT showed a 2.1-cm left pelvic lymphadenopathy and blastic lesions corresponding to the uptake on bone scan. Germline and somatic testing were negative for pathogenic alterations.
The patient started treatment with enzalutamide (Xtandi) taken orally at 160 mg every day. His PSA decreased to nadir of 3.9 ng/mL 4 months after starting enzalutamide, and his bone pain was resolved. After 8 months on enzalutamide, his PSA level was 12.6 ng/mL, but he was unavailable for intense PSA follow-up. Four months later, he returned with a PSA level of 48.1 ng/mL. Abdominal/pelvic CT showed enlargement of known pelvic lymph nodes. There was progressive disease on the bone scan with new lesions and he reported new back pain and progressive fatigue. His ECOG performance score was 1. He was now considered metastatic and castration resistant.
The patient then started on 75 mg/m2 of docetaxel given every 3 weeks plus 10 mg of prednisone a day. The patient clinically responded with resolution of pain and improved energy, with a declining PSA. However, with 4 cycles completed, the patient developed worsening bilateral digital neuropathy, so therapy was stopped before cycle 5. Three months later, he had a rising PSA, new back pain, and shortness of breath on exertion. Abdominal/pelvic CT scan now shows enlargement of known pelvic lymph nodes and 1 new liver lesion (less than 2 cm).
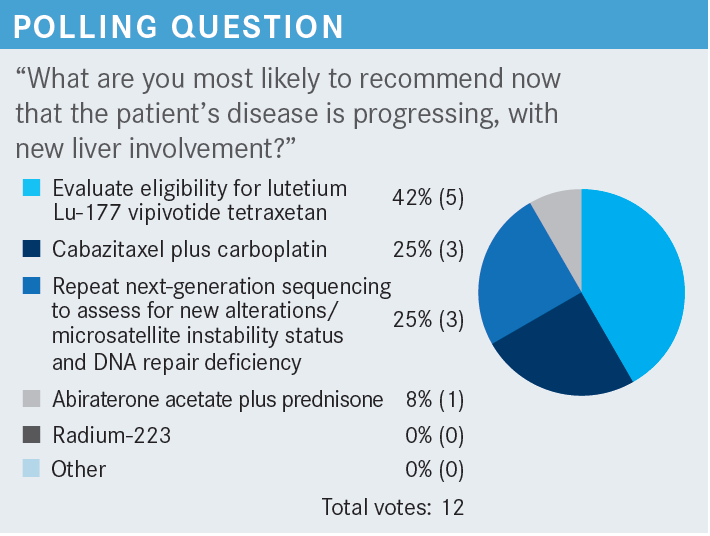
DISCUSSION QUESTIONS
- How do you select next line of therapy for a patient with mCRPC (metastatic castration-resistant prostate cancer) who has received docetaxel and an androgen receptor–targeted therapy?
- What factors do you consider when deciding on treatment?
NADIPURAM: In prostate cancer, there are so many options available. If the performance status is OK, it’s very reasonable to give second-line therapy. I would even try third-line therapy many times, but second-line therapy is very reasonable.
ZAKHARIA: It sounds like performance status is a major factor in your decision.
NADIPURAM: Correct.
KYRIAKOPOULOS: I think all [options] are probably the right answer. What I’m concerned about, especially in this case, is the new development of liver disease. When I see new liver metastasis, if the patient has a low PSA , it is a red flag. I typically repeat the biopsy to make sure there’s no small cell transformation or neuroendocrine transformation. I tend to be more aggressive with these patients.
ZAKHARIA: Absolutely.
KYRIAKOPOULOS: It’s great that we have lutetium Lu-177 vipivotide tetraxetan [Pluvicto], but I would typically treat with chemotherapy. Of course, in his case, neuropathy is a limiting factor. Docetaxel was discontinued after 4 cycles because of that reason, so another round of chemotherapy could potentially cause more neuropathy. But again, it’s a fine line. It’s a matter of what you pick, either a little more neuropathy or an inferior outcome.
ZAKHARIA: I agree. Indeed, liver metastasis is a red flag, especially when they have low PSA. For that same reason, they may have small cell or neuroendocrine [transformation]. That’s why I tend to biopsy, too. This patient did have a high PSA, which correlated with adenocarcinoma. But still, I would have a very low threshold for a repeat biopsy when we have the liver metastases, at least to achieve next-generation sequencing but mainly to rule out the small cell transformation. Have you seen a lot of small cell transformation in your practice?
KYRIAKOPOULOS: Quite a few. This past week, I had 2 patients with liver metastases and low PSA, so both were neuroendocrine transformation.
ZAKHARIA: Certainly, I have seen many cases, too.
CASE UPDATE
A decision was made to start cabazitaxel (Jevtana).
DISCUSSION QUESTION
- What starting dose of cabazitaxel are you most likely to use?
ZAHKHARIA: The approved dose was initially 25 mg, but then there was an inferiority study [PROSELICA; NCT01308580] that compared the 20 mg and found similar efficacy but better tolerability,1 so 20 mg tends to be the more commonly used regimen. Although the CHAARTED2 study [NCT03419234] used the 25-mg dose, too. But outside the clinical trial, 20 mg is the standard dose, if you will.
We give cabazitaxel with steroids and [granulocyte-macrophage colony-stimulating factor] support. It tends to have a better neuropathy profile, especially at 20 mg/m² compared with docetaxel. As we all know, it’s approved in post docetaxel at 20 mg/m². That’s the dose I would consider for my patients. You might consider 25 mg/m2 with steroids for healthy patients who wish to be more aggressive, but the data seem to support 20 mg/m2 being a sufficient dose, especially when combined with carboplatin.1
DISCUSSION QUESTIONS
- Would you give cabazitaxel to a patient who did not tolerate docetaxel well?
- If this patient had completed at least 6 cycles of docetaxel, would your recommendation differ?
- Have you used carboplatin plus cabazitaxel? If yes, please share your experience. In what scenario(s) did you use it?
NADIPURAM: I would test it. I would see how it does. If there is no progression of the neuropathy, it’s very reasonable to use it as a second-line option. It’s a good drug. I have seen good response with cabazitaxel, but neuropathy must be monitored.
ZAKHARIA: If this patient had completed at least 6 cycles of docetaxel, would your recommendation differ?
NADIPURAM: No, it doesn’t.
KURNIALI: I have some experience with cabazitaxel and carboplatin. [However], with the previous adverse effects [AEs] of docetaxel, if it’s primarily myelosuppression, it will be challenging. If it’s only neuropathy per se, I tend to challenge my patients with cabazitaxel, and some of them handle it quite OK. [However], myelosuppression or significant deconditioning would be a limiting factor.
ZAKHARIA: Certainly. I’ve seen those cases where it’s hard to revive the bone marrow, especially after prior taxane therapy.
DISCUSSION QUESTIONS
- Have the CARD trial (NCT02485691) data influenced your practice?
- What is your approach in managing toxicities associated with cabazitaxel?
- How do you counsel patients regarding infusion vs oral options and their tolerability profiles?
ZAKHARIA: Have you encountered a lot of patients who say they would rather stick to an oral pill rather than IV [intravenous] infusion? Do you prefer IV infusion, especially for those patients who might not be too reliable [adherence]- wise to take their oral pills at home? Can anybody share their experience with us?
FAHED: Most of our patients are on abiraterone acetate [Zytiga] and receive the triple therapy. They get the docetaxel upon diagnosis with metastatic hormone-sensitive prostate cancer, so most of them. If they progress on abiraterone, by default, cabazitaxel is going to be the next best option if they do have visceral disease plus or minus bone metastases. Otherwise, it would be radium-223 dichloride [Xofigo]. That has been my approach. I think the data are a little outdated right now.2 We use up-front abiraterone, enzalutamide [Xtandi], or apalutamide [Erleada], then move to radium-223 or cabazitaxel at the second onset.
ZAKHARIA: Great point. I agree that these data are a bit outdated now, and the field is moving so fast. With phase 1 trials, now we are using a triple combination in the [castration-sensitive prostate cancer] setting with maybe cabazitaxel or lutetium Lu-177 vipivotide tetraxetan for PSMA [prostate-specific membrane antigen]- targeted therapy.
I think it’s fine to extrapolate from the CARD trial data and other older clinical trial data that we have to help us best sequence these options, but you’re absolutely right. Every drug seems to be moving earlier during treatment, and sometimes we struggle with what would be the evidence-based medicine nowadays when the field is moving so fast, which is a good problem to have.
JI: I like the [CARD trial] data. Soon after they were published, I incorporated that into my practice. The response has been good, but I believe the trial used cabazitaxel until disease progression, right?2
ZAKHARIA: Correct.
JI: With time, patients will develop myelosuppression, cytopenia, and a lot of other AEs. [However], my only concern is for elderly patients. Can you share your experience? I have many patients in their late 80s. It’s easy to treat them in the first line. You give them abiraterone, then they have a good response for [approximately] 1 year, then they’re going to progress. For second-line therapy—like the control arm in this trial—if you give them anything like endocrine therapy, it’s not going to last too long, probably 2 or 3 months maximum. So that’s where we stop. But for patients in their late 80s with borderline status, how do you use cabazitaxel? I think this study did include patients up to 85 years, but now I’m just worried when I give it to patients in their late 80s.
ZAKHARIA: Yes, Dr Ji. I think you raise a great point. If your patients are like mine, who are Iowa farmers in their 80s, then I have [given cabazitaxel] to many of them in their late 80s here in the Midwest. I have patients who were 85 to 86 years old, who tolerated it better than even younger patients. [However], if they are very frail and in their 80s, I would counsel them very well about the chemotherapy. I would be very hesitant to consider it, especially if they have used chemotherapy in the past, because their bone marrow might not recover as well. Lutetium Lu-177 vipivotide tetraxetan can be another option, but the concern with bone marrow toxicity is there, too. Supportive and palliative care might be another option if they are too frail, but age has not been a deal breaker for me to use chemotherapy if they are in good shape.
JI: In terms of chemotherapy, do you prefer cabazitaxel more than docetaxel?
ZAKHARIA: I do not necessarily have a preference of one over the other. I try to get my patients on both. For docetaxel, we are using it very early on; a lot of my patients are receiving it in the CRPC setting. [By default], cabazitaxel will be [a] go-to early in the CRPC setting. [However], I do not try to compare which one is better than the other. We do not have strong comparison head-to-head studies to say one is better than the other. I tend to believe, because of the mechanism of action, that cabazitaxel might work in CRPC after docetaxel failure because of the [multidrug resistance], and that’s why the data and trial showed benefit after docetaxel.2
JI: Optimally, do you dose reduce? Do you use 20 mg/m2 as your starting dose?
ZAKHARIA: Yes, for almost all my patients, I do 20 mg/m2. If they do not handle it, I drop [the dose] by 25%.
DISCUSSION QUESTIONS
- Discuss real-world challenges with lutetium Lu-177 vipivotide tetraxetan in the community setting (eg, access, cost, time, insurance).
- What is your access to PSMA imaging and lutetium Lu-177 vipivotide tetraxetan? How feasible is it logistically for your patients?
ZAKHARIA: I think all of us believe that PSMA-targeted therapy, based on the VISION study [NCT03511664], is another good treatment option.3 The real-world challenge is mainly the shortage at the manufacturer level. [However], I’m curious to know from my colleagues in the community: Would you plan to implement this in your practice? I know many of our colleagues did not implement radium-223. Where do you see PSMA-targeted therapy, along with a required PSMA PET scan—the gallium Ga 68 gozetotide [Locametz] PSMA PET scan—and reimbursement and insurance?
FRONTIERA: As a community physician, we’ve been trying to get it at our hospital, but we’ve been put on hold.
ZAKHARIA: Is that because of reimbursement issues or shortages as well?
FRONTIERA: It is from the company saying they just don’t have enough supply. Every time we try to get an update, they say soon. We are doing the PSMA PET scans pretreatment, but we must refer them out for the actual therapy.
ZAKHARIA: Right.
FRONTIERA: [However], the company has promised [it will be available]…and we’ll see.
ZAKHARIA: I have heard similar answers. But luckily, we were able to treat many patients so far since the approval over the past couple months. We had access to the drug on-label, but now our patients are delayed 2 months out on a waiting list.
FLEJSIEROWICZ: I agree. It’s available; it’s out there, but many times, patients don’t want to wait. They just want to have something available to them, which is better than promising that there is this option but it will take a lot of time. They may opt out of it because they just don’t want to wait until something is available.
ZAKHARIA: In your experience, have you been bridging with more therapy?
FLEJSIEROWICZ: Yes.
ZAKHARIA: Until they get to that point?
FLEJSIEROWICZ: Exactly. It’s usually either the [ADT] or possibly chemotherapy until they get the drug.
ZAKHARIA: Have you had any success getting a patient to lutetium Lu-177 vipivotide tetraxetan?
FLEJSIEROWICZ: Not yet.
NADIPURAM: I have not used it, but in what line of treatment do you place this? After failure of cabazitaxel? Where does it play a role?
ZAKHARIA: The trial allowed 1 or 2 prior taxanes, so it was given before or after cabazitaxel failure. It required prior hormonal therapy, so after any hormonal [therapy] and chemotherapy is when we are considering this.
NADIPURAM: Are you using ADT?
ZAKHARIA: Yes.
NADIPURAM: When you are using it?
ZAKHARIA: ADT remains at the baseline.
BAE: It looks like lutetium Lu-177 vipivotide tetraxetan is limited, so it’s very important to pick the right patient for this. Are PSMA SUVmax [maximum standardized uptake values] greater than 20 at any sites and no [18F-fluorodeoxyglucose]-avid or PSMA-negative sites of disease the criteria you use in selecting lutetium Lu-177 vipivotide tetraxetan treatment? [Also], who does this treatment? Is it radiation oncologists or you?
ZAKHARIA: This is a great question. Different trials have used different PSMA SUVmax values. Some of them have used 10 or 20. At our institution, we work very closely with our radiation oncologists and our nuclear medicine team, so they offer the drug. We refer the patient to them, and we watch them together.
We have established a lutetium Lu-177 vipivotide tetraxetan clinic for patients who come multiple times. We have a dedicated team who handle those patients, and we have established an order set in [our ordering system], along with baseline-required scans for insurance satisfaction.
In my experience, all insurance companies—or [most]— have been mandating a gallium Ga 68 gozetotide PSMA PET scan. We have had a few patients get the piflufolastat F 18 PSMA PET scan [Pylarify], which is a different service, and the insurance will decline that. They require a gallium Ga 68 gozetotide PSMA PET scan [based on the FDA approval].
FRONTIERA: I’m a little surprised that there’s no difference in overall survival [OS], considering the responses, especially with the tail on the curve. Why do you think that’s the case?
ZAKHARIA: It was the TheraP trial [NCT03392428] that did not show a survival benefit,4 but the VISION study showed a preference toward PSMA over hormonal therapy from an OS standpoint.3 We need longer follow-up for mature data. But the TheraP study that compared PSMA with cabazitaxel did not show an OS benefit.
FRONTIERA: That’s a little surprising. Is it just because it’s early?
ZAKHARIA: One might argue that maybe cabazitaxel is a better control arm in this setting, simply because we know it works after docetaxel as opposed to hormonal sequencing back-to-back, which might not be the best strategy. Nevertheless, it’s still a 200-patient study, 100 per arm. It might be a little too small to show a robust difference in OS, plus, many of those patients had subsequent therapy. If they got cabazitaxel, they went onto lutetium Lu-177 vipivotide tetraxetan and vice versa,4 so that is another confounding factor that might have impacted the OS.
KYRIAKOPOULOS: Overall, it’s very difficult to show survival benefit for third-line treatment and beyond. Patients who progress on lutetium Lu-177 vipivotide tetraxetan could potentially receive cabazitaxel.
ZAKHARIA: Correct.
KYRIAKOPOULOS: It’s very difficult. I think it’s the right thing that both the TheraP and the VISION studies didn’t include OS as the primary end point. I think it was the right thing from the FDA standpoint that they didn’t approve lutetium Lu-177 vipivotide tetraxetan based on the OS data, but it was based on the [radiographic progression-free survival] benefit.5
ZAKHARIA: Yes, I agree. Typically, by the time they make it to third-line treatment, patients have seen [most] of the classes of drugs, [which is] why OS gets confounded in this late stage of treatment.
REFERENCES
1. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35(28):3198-3206. doi:10.1200/ JCO.2016.72.1076
2. de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506- 2518. doi:10.1056/NEJMoa1911206
3. Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
4. Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804. doi:10.1016/S0140-6736(21)00237-3
5. FDA approves Pluvicto for metastatic castration-resistant prostate cancer. News release. FDA. March 23, 2022. Accessed March 9, 2023. https://bit. ly/4204F9f