Kim Discusses Data for Chemotherapy and Immunotherapy in Biliary Tract Cancers
During a Targeted Oncology™ Case-Based Roundtable™ event, Richard Kim, MD, discussed regimens for biliary tract cancers including the results of the TOPAZ-1 trial of additional durvalumab.

Richard Kim, MD
Professor of Oncology
University of South Florida College of Medicine
Service Chief of Medical Gastrointestinal Oncology
Moffitt Cancer Center
Tampa, FL

Targeted OncologyTM: What are the subtypes and current incidence of biliary tract cancer (BTC)?
KIM: BTC is a heterogeneous disease. It includes intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma, and extrahepatic cholangiocarcinoma could be divided into perihilar and distal [subtypes]. There is also gallbladder cancer and the risk factors are all different. If you molecularly profile all these patients, then molecular profiling phenotypes are different in all 3 types, even though for [patients with] advanced disease we treat them the same.
If the disease is locally advanced and [treated] early, the treatment will be different in terms of surgical resection along with, potentially, somewhat targeted therapy because the actual mutation is different [in the different disease categories]. For example, in intrahepatic cholangiocarcinoma you see a lot of FGFR2 features and IDH1 mutations. For extrahepatic cholangiocarcinoma, you may see more KRAS mutations. For gallbladder cancer, you may see more HER2 amplification.1 This is a heterogeneous [disease], and we have to treat the [types] differently and profile them accordingly. In terms of incidence, [BTCs] are on the rise, mostly intrahepatic cholangiocarcinoma. Many years ago, intrahepatic cholangiocarcinoma was called a cancer of unknown primary, but now, with better imaging, better diagnostics, better pathology, with profiling of the tumor, this cancer of unknown primary [is being identified as] intrahepatic cholangiocarcinoma.
Those are the patients that sometimes get referred to Moffitt Cancer Center for unknown primary. When they do the workup, it’s intrahepatic cholangiocarcinoma. Unfortunately, the 5-year overall survival [OS] for this disease is very poor, less than 20%. Even after resection, the tumor tends to come back, and about 70% of patients present at an advanced stage.2
What are the risk factors for the different types of BTC?
With gallstones, you have risk of developing cancer in the gallbladder, but [you are also at risk for developing BTC] if you have diabetes, biliary tract infection, obesity, or pregnancy. In Southeast Asia, liver flukes are the No. 1 reason for why you see a lot of cases of cholangiocarcinoma.
Primary sclerosing cholangitis and primary biliary cirrhosis are 2 risk factors of developing cholangiocarcinoma. Another risk factor that may not be well known is hepatitis C. We know that hepatitis C cirrhosis is a risk factor for developing hepatocellular carcinoma, but it is also a risk factor for developing cholangiocarcinoma.
What are the signs, symptoms, and workup that lead to the diagnosis of BTC?
The symptoms are very vague. You may have some pruritus, yellowing jaundice, pain, fever, and weight loss and depending on where the tumor is, the signs will vary. If it is a perihilar tumor, the patient may present with painless jaundice, but if it is an intrahepatic cholangiocarcinoma, they may just present with pain or itchy skin. It depends on the location of the tumor. The work-up will depend on what you find, but the workup is pretty straightforward. It will include laboratory tests, imaging, and pathology.
Intrahepatic cholangiocarcinoma is almost a diagnosis of exclusion. When you see disease in the liver and you biopsy it and it comes back as adenocarcinoma that is CK7 positive and CK20 negative, you have to do the other workup to make sure there’s no other primary [tumor]. Once you do a CAT scan and [find that] there are no other sites of disease and [that it is] mostly confined to the liver, based on the immunohistochemistry and the radiographic finding, [you can] diagnose an intrahepatic cholangiocarcinoma. The [molecular] profiling of the tumor helps a lot, too.
How are patients with advanced BTCs currently treated?
At Moffitt, we probably see [more than] 200 patients per year with cholangiocarcinoma. The [National Comprehensive Cancer Network] guidelines for first-line treatment include gemcitabine plus cisplatin.3 This regimen was based on the results of the ABC-02 study [NCT00262769] that has been the standard for many years. Then the TOPAZ-1 [NCT03875235] data came out and that study looked at durvalumab [Imfinzi] plus gemcitabine and cisplatin.
For second-line treatment, if you have actionable mutations—for example, a FGFR fusion or IDH1 mutation—[the guidelines offer targeted regimens, either pemigatinib (Pemazyre) plus futibatinib (Lytgobi) or ivosidenib (Tibsovo), respectively]. If the patient does not have any actionable targets, then the second line will most likely be the combination of oxaliplatin [Eloxatin], folinic acid, and 5-fluorouracil, and there are some data [on the NIFTY trial; NCT03524508] of the regimen of 5-fluorouracil plus liposomal irinotecan [Onivyde].4 That’s mostly from Asia; I would say those data [from NIFTY] are very controversial.... If the patient has a BRAF mutation, you have the combination [of dabrafenib (Tafinlar) plus trametinib (Mekinist)].
For NTRK fusion, which is very rare—I have not yet seen an NTRK fusion with cholangiocarcinoma—there is [larotrectinib (Vitrakvi)]. There is pembrolizumab [Keytruda] if the patient has high microsatellite instability [MSI-H] or mismatch repair deficiency. And if the patient did not get immunotherapy in the first line, the last option is gemcitabine, cisplatin, and nab-paclitaxel [Abraxane].5
What were the results of the ABC-02 study of gemcitabine plus cisplatin?
This was a phase 3 study published about 12 years ago, which showed that compared [with] gemcitabine alone, gemcitabine plus cisplatin increased OS and progression-free survival [PFS] by a couple of months. The schema of the study was gemcitabine plus cisplatin for about 8 cycles— 6 months—followed by nothing. They would just do close surveillance, and when a patient would relapse, they would introduce chemotherapy again.6
The subgroup analysis of the ABC-02 study showed that most patients did benefit from the combination. If there is 1 exception, [it would be] patients with an ECOG performance score of 2 as those patients had a borderline benefit. Otherwise, most of the patients did benefit from this combination, whether a patient’s primary tumor was intrahepatic, extrahepatic, or in the gallbladder. Interestingly, [in] this study they [included] ampulla of Vater as a primary tumor site. There were only 20 such patients, but the study did include them.6
What has been shown about the efficacy of gemcitabine, cisplatin, and nab-paclitaxel?
Those data were impressive—though I want to point out that these are phase 2 data—with a median PFS of [11.8] months [95% CI, 6.0-15.6] and a median OS of 19.2 months [95% CI, 13.2-not estimable].7 The partial response rate was [45%], and many of these patients were downstaged and would go for resection.7 Having said that, we know that once you start adding a third chemotherapy, you are concerned about toxicity. So even though the schedule is day 1 and day 8, every 21 days, it’s tough to give those triplet regimens on back-to-back weeks because of health issues. Sometimes I do every 2 weeks or cut down the dose.
They looked at OS by dose and by tumor type. There might have been some correlation between dose [and OS], so dose intensity may matter [median OS, 19.5 months vs 15.7 months for high dose and reduced dose, respectively]. The patients who benefited most were the patients with intrahepatic cholangiocarcinoma [median OS, not estimable vs 13.2 months vs 15.7 months for intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and gallbladder cancer, respectively].7 Is that surprising? No, because in the results of the ABC-02 study, the median OS in patients who got gemcitabine plus cisplatin was close to 17 or 18 months.8 And by far, the patients with intrahepatic cholangiocarcinoma did the best, followed by those with gallbladder cancer, and followed by those with extrahepatic cholangiocarcinoma.7
PFS [was also analyzed with respect to dose and] tumor type, and the results were mixed. Once again, these are phase 2 data. The reduced-dose patients had longer PFS [14.9 months vs 11.4 months for high-dose patients]. Maybe they were able to tolerate the drug better with a lower dose, but, as expected, the median PFS of patients with intrahepatic cholangiocarcinoma was over 1 year vs 6 months for those with extrahepatic cholangiocarcinoma and [4.1 months] for those with gallbladder cancer.7
What comprises the body of work investigating immune checkpoint inhibitors in the treatment of BTC?
Immune checkpoint inhibitors have been studied as single agents and as doublets, mostly in the refractory setting. Some of the trials that were conducted using a single agent investigated pembrolizumab and nivolumab [Opdivo]. Nivolumab plus ipilimumab [Yervoy] and durvalumab plus tremelimumab [Imjudo], and other combinations, were investigated as well. But as a single agent or as a doublet, the response rates were modest, hovering around 5% to 15%, [except for] the patients who had MSI-H status, in which case the response rate was much higher.
Nivolumab plus ipilimumab seems to have had a little higher response rate in the refractory setting as well.4 But generally speaking, cholangiocarcinoma is what we call a lukewarm tumor; it’s not a hot tumor and you don’t expect to see a huge response with a single-agent immunotherapy. However, if you combine it with chemotherapy, that may change the microenvironment, allowing the therapy to work better, and patients with MSI-H status or mismatch repair deficiency respond very nicely to immunotherapy.
Could you describe the trial that led to the approval of the addition of durvalumab to gemcitabine/ cisplatin for BTC?
The TOPAZ-1 study was in patients with locally advanced [or metastatic] BTC tumors. [They must have] previously had no systemic treatment [if they were unresectable or metastatic at diagnosis], and if they recurred up to 6 months after curative surgery or adjuvant therapy, those patients were allowed. They were randomly assigned 1:1 to [receive] gemcitabine/cisplatin plus durvalumab up to 8 cycles, followed by durvalumab maintenance every 4 weeks afterward or to the other arm, which [was] gemcitabine/cisplatin for 8 cycles followed by placebo. In the ABC-02 study, after 8 cycles, they were done. Even though in our practice we [may] put them on maintenance therapy, that was not done for the ABC-02 study. The primary objective [of TOPAZ-1] was OS, and secondary objectives were PFS, ORR, and duration of response.
If you look at the demographics, I want to point out that... [more than] 50% of the patients were enrolled in Asia, which is very important.9 But…the 2 arms are very well balanced [by region]. The second thing is that most of the tumors were intrahepatic cholangiocarcinoma, which if it were done in the United States may have been higher than 55%, maybe even 65%. They did look at MSI status because we expect patients who [are] MSI-H to respond better. Luckily, there [were] only a few that were MSI-H to begin with, 2 or 3 patients in each arm. Most had MSI-stable status; however, [51.4%] of the patients were missing [MSI status].
I don’t think they’ve reported the tumor mutational burden, at least in this paper. They also noted the viral etiology, which is important because there’s a hypothesis on correlation between the viral etiology and immunotherapy. But in this case, since this is not hepatocellular carcinoma, [approximately] 50% had no viral hepatitis. However, [approximately] 20% had hepatitis B, which is expected because 50% of the patients were from Asia. There were very few patients with hepatitis C [and] also 24.1% whose hepatitis profile was missing.
What were the efficacy outcomes of this trial?
If you look at the primary end point of the study, this was a positive study, [with] a median OS of 12.8 months with durvalumab vs 11.5 months with placebo with a hazard ratio of 0.80 [95% CI, 0.66-0.97; P = .021]. But on the Kaplan- Meier curve, there’s a clear separation [after 23 months].9
If you look at the subgroup analysis for OS, depending on the sex [of the patient], the PD-L1 expression, intrahepatic [vs extrahepatic or gallbladder], Asian vs non-Asian, every [group] benefited. The combination of durvalumab plus gemcitabine/cisplatin [appeared to be] independent of any subgroup patients fell under. Overall, it seems like most of the groups did benefit from the combination.9
If you look at the PFS, there was improvement in PFS from 5.7 months with placebo to 7.2 months with durvalumab [HR, 0.75, 95% CI, 0.63-0.89; stratified log-rank P = .001]. The beauty of immunotherapy is that there’s always a [tail]; the OS Kaplan-Meier curve [had one], since there are certain patients that truly do benefit from immunotherapy because they are very long-term survivors. In PFS...you see a tail as well, where there are some patients, 20% to 25%, who do benefit tremendously from combination with immunotherapy.9
What were the safety and tolerability observed in this trial?
In terms of the safety data, we noticed that by adding immunotherapy to gemcitabine/cisplatin, there was overall not too much difference in terms of adverse events [AEs]. If you look at the treatment-related AEs and for any-grade or for serious AEs, you cannot tell the difference which patients were getting what in terms of whether they were getting placebo or durvalumab with combination gemcitabine/ cisplatin [Table9].
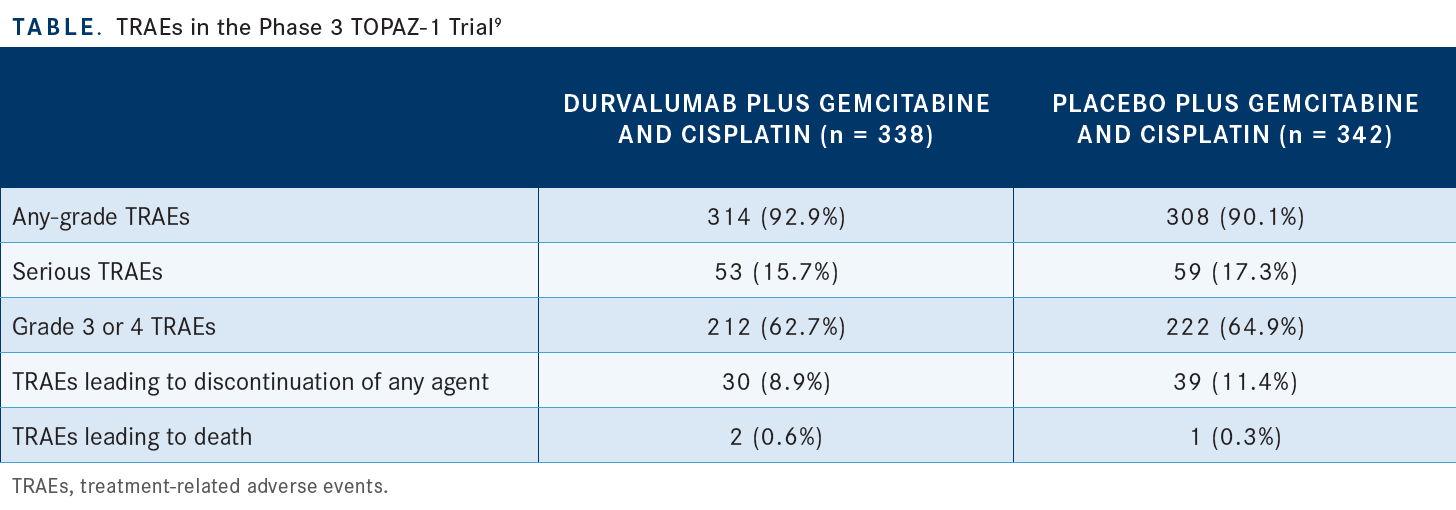
Grades 3 and 4 were [62.7% with durvalumab] vs [64.9% with placebo].9 Serious AEs occurred in 15.7% with durvalumab vs 17.3% with placebo.9 Overall, by adding durvalumab to gemcitabine/cisplatin, you’re fortunately not getting a lot of toxicity out of it.
REFERENCES
Haber PK, Sia D. Translating cancer genomics for precision oncology in biliary tract cancers. Discov Med. 2019;28(155):255-265.
Lamarca A. Current landscape in biliary tract cancers. Presented at: 2022 American Society of Clinical Oncology Gastrointestinal Cancers Symposium; January 20-22, 2022; San Francisco, CA. Accessed March 1, 2023. https://bit. ly/3M1bv99
NCCN. Clinical Practice Guidelines in Oncology. Hepatobiliary cancers, version 5.2022. Accessed March 2, 2023. https://bit.ly/3G5kIJT
Yoo C, Kim KP, Jeong JH, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021;22(11):1560-1572. doi:10.1016/S1470-2045(21)00486-1
Cowzer D, Harding JJ. Advanced bile duct cancers: a focused review on current and emerging systemic treatments. Cancers (Basel). 2022;14(7):1800. doi:10.3390/cancers14071800
Valle J, Wasan H, Palmer DH, et al; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. doi:10.1056/NEJMoa0908721
Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5(6):824-830. doi:10.1001/jamaoncol.2019.0270
Lamarca A, Ross P, Wasan HS, et al, Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst. 2020;112(2):200-210. doi:10.1093/jnci/djz071
Oh DY, He AR, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8). doi:10.1056/ EVIDoa2200015