Langer Assesses Several Targeted Treatment Options in the Non–Small Cell Lung Cancer Landscape
During a Targeted Oncology™ Case-Based Roundtable™ event, Corey J. Langer, MD, discussed the EMPOWER Lung-3 and POSEIDON trials of immunotherapy options in patients with non–small cell lung cancer.
Corey J. Langer, MD
Director, Thoracic Oncology
Professor of Medicine
Hospital of the University of Pennsylvania
Penn Medicine-Abramson Cancer Center
Philadelphia, PA

Targeted OncologyTM: What molecular- and biomarker-directed regimens are preferred for the first-line treatment of advanced or metastatic non– small cell lung cancer (NSCLC), according to the guidelines of the National Comprehensive Cancer Network (NCCN)?
LANGER: The preferred regimen for nonsquamous NSCLC is histology-specific chemotherapy, that is, carboplatin [or cisplatin] with pemetrexed [Alimta] and pembrolizumab [Keytruda]. For [treating patients with] squamous NSCLC, the preferred regimen is carboplatin plus solvent-based paclitaxel or albumin-bound nab-paclitaxel [Abraxane] plus pembrolizumab. But in 2022 [there were] some additions. These include cemiplimab-rwlc [Libtayo] and histology-specific chemotherapy, and the POSEIDON [NCT03164616] approach, which is like the CheckMate 9LA [NCT03215706] regimen…tremelimumab-actl [Imjudo] plus durvalumab [Imfinzi] plus chemotherapy, not for 2 cycles but for 4 cycles, with maintenance pemetrexed, at least in the nonsquamous population.1 So our array of options is expanding.
What data supported the approval of these latter 2 regimens?
The FDA approvals for cemiplimab-rwlc plus platinum-based chemotherapy and the combination of tremelimumab, durvalumab, and platinum-based chemotherapy came 2 days apart. The approval for the cemiplimab combination was based on phase 3 data, specifically in patients with no evidence of EGFR, ALK, or ROS1 molecular aberrations.
The study included not just patients with metastatic disease but also those with locally advanced disease who were not candidates for surgical resection or definitive chemoradiation.2 Then the FDA approved the POSEIDON approach, which combined a CTLA-4 inhibitor with durvalumab and platinum-based chemotherapy, again with no antecedent EGFR or ALK genomic alterations.3
What study explored the use of pembrolizumab along with carboplatin and paclitaxel?
KEYNOTE-407 [NCT02775435] [studied this combination in] a 1:1 random assignment. The control arm was placebo and carboplatin plus either standard solvent-based paclitaxel given every 3 weeks or nanoparticle albumin-based paclitaxel, generally my preferred combination, given weekly. Off study, I generally give it on day 1 and day 8 and omit day 15.
This regimen was compared with the same combination with pembrolizumab, with the pembrolizumab continued for up to 2 years. Note that there was optional crossover in the control arm to single-agent pembrolizumab at the time of progression, which was strictly in squamous-cell NSCLC. Molecular markers were not required as part of the analysis, and stratifications were based on PD-L1 status, the choice of taxane, and the region of the world. The primary end points in that study were progression-free survival [PFS] and overall survival [OS].4
What was the objective of the CheckMate 9LA study?
This study essentially compared chemotherapy alone to ipilimumab [Yervoy] plus nivolumab [Opdivo] plus 2 cycles of histology-specific chemotherapy. Stratifications were based on PD-L1 status and histology. The primary end point, as it should be in all of these studies, was OS. In this study, as with pretty much all CheckMate and KEYNOTE studies, EGFR and ALK [mutations] were excluded.5
What were the results of the KEYNOTE-407 and CheckMate 9LA studies?
The updated results are standing the test of time. The median OS in both of these studies improved by approximately 5 to 6 months. That’s about 6% to 8% better compared with the control arm [median OS in the experimental arm, 17.2 months (HR, 0.71; 95% CI, 0.59-0.85) in KEYNOTE-407 and 15.8 months (HR, 0.72; 95% CI, 0.61-0.86) in CheckMate 9LA]. The control arms did better than they’ve done historically in part because they’re allowed to cross over to immunotherapy. Virtually every patient experienced some adverse event [AE]. The treatment discontinuations were a bit more common for the immunotherapy combination but were still probably within the range of what we would tolerate clinically. There was a major improvement in objective response rates [ORRs], particularly for the KEYNOTE-407 approach [ORR, 62.2% vs 38.8% for pembrolizumab vs placebo, respectively].6,7
What were the results of the study that explored the cemiplimab plus platinum doublet regimen?
EMPOWER-Lung 3 [NCT03409614] was essentially [the regimens of] KEYNOTE-407 and KEYNOTE-189 [NCT02578680], combined, vs chemotherapy alone. It was placebo controlled, with the placebo combined with investigator’s choice of platinum doublet [therapy]. This placebo arm was compared to cemiplimab, given at a fixed dose of 350 mg every 3 weeks, plus investigator’s choice of platinum doublet, presumably histology appropriate.
Patients were excluded if they had EGFR, ALK, or ROS1 mutations. Stratifications were based on PD-L1 expression less than 1%, 1% to 49%, or 50% or higher, and by histology. The primary end point was OS. Pemetrexed maintenance was mandatory for patients whose disease had nonsquamous histology and treatment continued for a full 2 years.8
In this study, nonsquamous disease was in the majority at approximately 57%. The vast majority of patients had at least some symptoms, and [84.3%] had an ECOG performance status of 1.8 The demographics were similar to what we’ve seen in the other studies.4,5,8 A small minority, only [14.4%], were never smokers. Some had had prior therapy, usually adjuvant [1.3%] or radiation [10.9%]. Men, particularly outside the US, do tend to predominate on these clinical trials [in this study, comprising 83.9% of the population]. There was a roughly equal distribution of PD-L1 expression among the 3 subgroups, and that pretty much mirrors what we saw in the KEYNOTE-407 and KEYNOTE-189 trials.4,8
There was a nearly 9-month improvement in OS [at 21.9 months vs 13.0 months for the experimental and control arms, respectively]. Remember, this was in patients with both squamous and nonsquamous histology, combined. At 1 year, the rate of OS was [65.7% vs 56.1% in the experimental and control arms, respectively (HR, 0.71; 95% CI, 0.53-0.93; P = .014)], an approximately 10% absolute difference.8 The HR was in line with that seen in the other studies.4,5,8 The trial was stopped early by the data monitoring committee because of the preset OS efficacy criteria.8
Nearly every subgroup enjoyed some [OS] benefit. Women were underrepresented in this trial and, for whatever reason, may not have benefited as much as did men. This was a hypothesis-generating [analysis], at most. I think there was a high concentration of never-smokers in that [study], and [they] did not have the same degree of benefit [as did smokers]. There was a greater benefit in patients with PD-L1 expression of 1% or higher, relative to those with expression of less than 1%. The HR favoring cemiplimab was 0.52 and 0.61, respectively, for patients with PD-L1 expression of under 1% to 50% and of 50% or higher.8
The PFS was in keeping with the OS data, with a nice separation at the median, an approximately 3-month advantage [8.2 months vs 5.0 months for the experimental and control arms, respectively; HR, 0.56; 95% CI, 0.44-0.70; P < .0001]. That separation occurred fairly early, at about 4 to 8 months and at 1 year there was more than a doubling in PFS, [38.1% vs 16.4%, respectively].8 According to the subgroup analysis of PFS, pretty much every group, with the possible exception of women, had a PFS advantage, regardless of race, histology, PD-L1 level, performance status, region of the world, presence or absence of brain metastases, stage, or smoking status.8
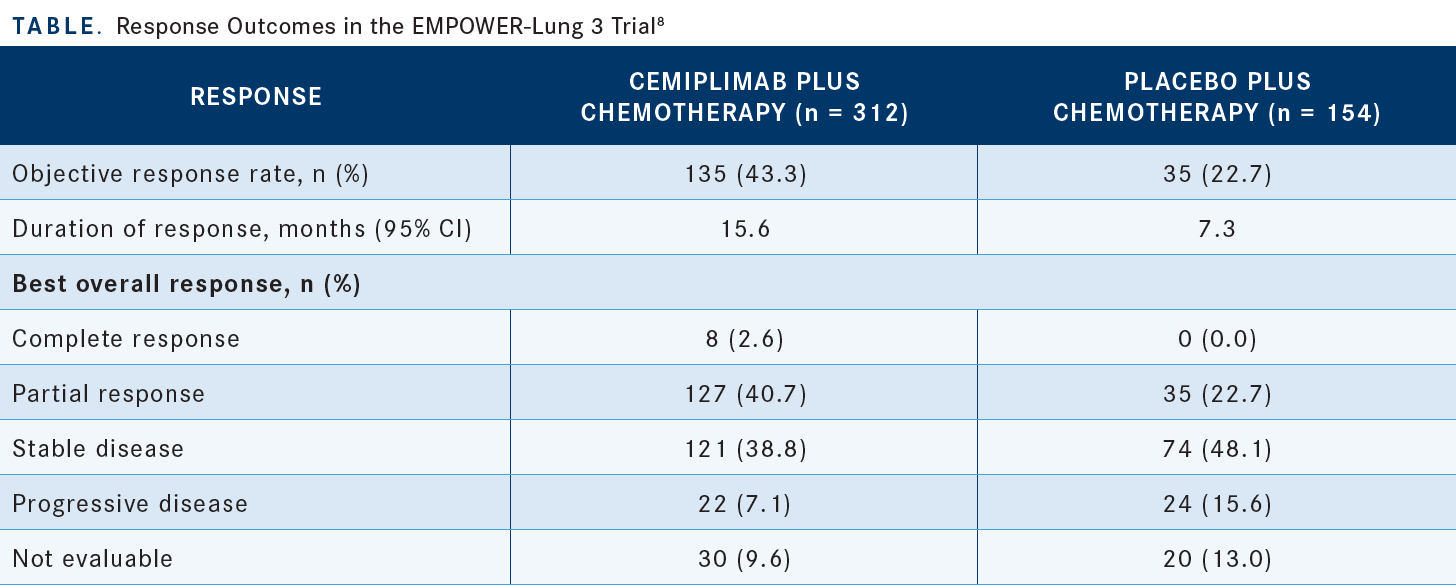
The ORRs were 43.3% vs 22.7% for the experimental and control arms, respectively. The rates of stable disease were [38.8% vs 48.1%], respectively. So the disease control rates were comparable between the arms, but the actual rate of response was clearly higher [in the experimental arm]. Also, the duration of response was twice as long in the experimental arm relative to the control arm [Table8].
As you’d expect, treatment-related AEs of grade 3 or higher were a bit more pronounced in the cemiplimab group than in the control group, [28.8% vs 18.3%, respectively], but there was no significant increase in treatment-related AEs of grade 5 [1.3% vs 0.7%, respectively]. There were a bit more that led to treatment discontinuations [of grade 3 or higher, affecting 2.2% vs 0.7%, respectively]. Immune-mediated AEs occurred in [18.9%] of patients in the experimental arm, but only [2.9%] were of grade 3 or higher.8
What were the design and results of the POSEIDON study of the addition of durvalumab, with or without tremelimumab, as first-line chemotherapy?
Platinum-based chemotherapy was given for up to 6 cycles, and this was compared with [a similar] approach as CheckMate 9LA, but with durvalumab and tremelimumab as opposed to nivolumab and ipilimumab.
The crucial difference here was that chemotherapy continued for 4 cycles and then patients could potentially have maintenance pemetrexed continue beyond that in nonsquamous disease. There was a third arm that looked at durvalumab alone, and the fact that we don’t talk about that arm [shows its lack of] efficacy.9 The eligibility criteria were very similar to those of the other trials; EGFR and ALK mutations were excluded.4,5,8,9 Over 1000 patients accrued to this trial, with the major end points being PFS and OS and durvalumab was given at a fixed dose, as was tremelimumab, every 3 weeks.9 For just the arms of chemotherapy alone vs the durvalumab, tremelimumab, and chemotherapy combination, just over one-third of the patients, approximately 37%, had no PD-L1 expression. Roughly 10% had central nervous system metastases, over 20% had liver metastases, and the vast majority, two-thirds, had an ECOG performance status of 1. Almost two-thirds of the patients had nonsquamous disease and, as is typical in these trials, only approximately 20% were never-smokers.9
The PFS analysis showed an advantage with the experimental regimen, with a median PFS of 6.2 months vs 4.8 months in the experimental and control arms, respectively [HR, 0.72; 95% CI, 0.60-0.86; P = .0003]. The PFS was doubled at a year, [26.6% vs 13.1%, respectively], with separation occurring at about 6 to 7 months.9 This, in turn, translated into OS advantage, and the data are fairly mature at this point.
At 4 years, survival was [20.7% vs 8.3%] in the experimental and control arms, respectively. Median OS values weren’t quite so different [14.0 months vs 11.7 months, respectively; HR, 0.75; 95% CI, 0.63-0.88], but the separation occurred beyond the median during the maintenance phase of immunotherapy. With the exception of never-smokers...there was a consistent OS advantage across nearly every demographic group.
One note regarding the KRAS-mutant population: The HR for this group was even better [than the overall population], at 0.55, with 3-year survival of [40.0% vs 15.8%, respectively].10 The AEs were a bit more pronounced in the immunotherapy combination arm. Treatment-related serious AEs affected [27.6%] in the triplet arm, [19.5%] in the arm with durvalumab plus chemotherapy, and 17.7% in the arm with chemotherapy alone.
The rate of AEs leading to death was not significantly higher in the triplet vs the control arm, 3.3% vs 2.4%, respectively. Immune-mediated AEs were obviously a lot higher in the triplet arm than in the control [33.6% vs 5.1%, respectively], but those of grade 3 and 4 affected only 10% of those in the triplet arm.10
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Non–small cell lung cancer, version 2.2023. Accessed March 7, 2023. https://bit.ly/3FcIOlv
2. FDA approves cemiplimab-rwlc in combination with platinum-based chemotherapy for non-small cell lung cancer. FDA. November 8, 2022. Accessed March 7, 2023. https://bit.ly/3ywbt0M
3. FDA approves tremelimumab in combination with durvalumab and platinum-based chemotherapy for metastatic non-small cell lung cancer. FDA. November 10, 2022. November 18, 2022. Accessed March 7, 2023. https://bit.ly/3T5Hd6x
4. Paz-Ares L, Luft A, Vicente D, et al; KEYNOTE-407 Investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040-2051. doi:10.1056/NEJMoa1810865
5. Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211. doi:10.1016/S1470-2045(20)30641-0
6. Novello S, Kowalski DM, Luft A, et al. 5-year update from KEYNOTE-407: pembrolizumab plus chemotherapy in squamous non-small cell lung cancer (NSCLC). Ann Oncol. 2022;33(suppl 7):S448-S554. doi:10.1016/annonc/annonc1064
7. Reck M, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open. 2021;6(5):100273. doi:10.1016/j.esmoop.2021.100273
8. Gogishvili M, Melkadze T, Makharadze T, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med. 2022;28(11):2374-2380. doi:10.1038/s41591-022-01977-y
9. Johnson ML, Cho BC, Luft A, et al; POSEIDON Investigators. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol. 2023;41(6):1213-1227. doi:10.1200/JCO.22.00975
10. Johnson ML, Cho BC, Luft A, et al. Durvalumab (D) ± tremelimumab (T) + chemotherapy (CT) in 1L metastatic (m) NSCLC: overall survival (OS) update from POSEIDON after median follow-up (mFU) of approximately 4 years (y). Ann Oncol. 2022;33(suppl 7):S808-S869. doi:10.1016/annonc/annonc1089