Roundtable Discussion: Khan Reviews Systemic Therapy for Relapsed/Refractory DLBCL
During a Targeted Oncology™ Case-Based Roundtable™ event, Cyrus Khan, MD, discussed the case of a patient with relapsed/refractory diffuse large B-cell lymphoma who was referred for chimeric antigen receptor T-cell therapy and was place on a waiting list for treatment.
Cyrus Khan, MD
Hematologist
Allegheny Health Network Cancer Institute
Pittsburgh, PA


CASE SUMMARY
A 43-year-old woman presented with fatigue and worsening, burning back pain. She had a medical history of mild hypertension that was well controlled with medication and her physical exam showed a 1.5-cm left posterior cervical node, a 2.5-cm right anterior cervical node, and a 2.0-cm left supraclavicular node. A CT scan of multiple enlarged mesenteric and retroperitoneal nodes showed that the largest measuring was 5.3 cm × 3.1 cm.
Biopsy confirmed diffuse large B-cell lymphoma (DLBCL) and her immuno-histochemistry was positive for:
- CD20
- BCL-6
- BCL-2 (50% of cells)
- MYC (> 90% of cells)
- Ki-67 (85% of cells)
- Normal complete blood count
Her lactate dehydrogenase was within normal limits and she had an ECOG performance status of 0. Fluorescence in situ hybridization was negative for chromosomal abnormality, so R-CHOP (rituximab [Rituxan], cyclophosphamide, doxorubicin, vincristine, and prednisone) was initiated for 6 cycles and back pain resolved. Cerebrospinal fluid was negative for lymphoma and a posttreatment PET scan demonstrated a complete response (CR) with a Deauville score of 2.
DISCUSSION QUESTIONS
- Do you ever consider another line of systemic therapy prior to the use of chimeric antigen receptor (CAR) T-cell therapy?
- Do you have a sense of how long it takes between T-cell collection and infusion in your practice region?
KHAN: Have you communicated with or worked with a CAR T center and then used certain therapies before the patients go on to cell collection, or even used bridging therapy?
BARSOUK: I’m on the referring side, but if CAR T-cell therapy is not available now, while waiting it would not be uncommon to use a second-line therapy until the CAR T-cell therapy becomes available, then after collection of T cells, while the product is manufactured, that [second-line therapy] would be used. Unlike with stem-cell transplant, you don’t need a strong CR or very good partial response. I don’t think it’s required, correct me if I’m wrong, for CAR T-cell therapy. So when it becomes available, the patient can proceed, right?
KHAN: Yes, you are right. You don’t need to push the patient into a CR; you just need to control the disease so it’s not blowing up before we have the opportunity for all those things. Typically, what I’ve seen in the community and [in my prior experience] would be drugs like gemcitabine [Infugem] plus oxaliplatin [Eloxatin; GemOx], and the newer therapies as well, of course.
FAROUN: To the question of if the patient is waiting for almost 2 months for CAR T-cell therapy, how to cool the disease off, it depends on the transplanter or the CAR T-cell center. Usually, I cool them off with either GemOx or even the anti-CD19 drug tafasitamab-cxix [Monjuvi].
I would like to use tafasitamab without lenalidomide [Revlimid] as lenalidomide might affect the recovery. Polatuzumab vedotin-piiq [Polivy] also could be used, but I don’t know if you have any other options and these are the new medications that I use for bridging.
KHAN: Exactly, you are doing it right. Do you have an idea of how long it takes, over there, from T-cell collection to the infusion?
FAROUN: A year ago, we had a hard time, and recently we might have it within 30 to 45 days of collection.
CASE UPDATE
Eleven months after completion of therapy, the patient complained of fever, night sweats, and back pain. A palpable lymph node in left groin was discovered on physical examination. Another PET/CT scan showed a new left inguinal lymph node, increase in size of residual node, as well as multiple metabolically active lesions in lymph nodes of the retroperitoneum, abdomen, and pelvis.
A biopsy revealed DLBCL and the patient was referred to a transplant center. R-ICE (rituximab, ifosfamide (Ifex), carboplatin, and etoposide) salvage therapy was initiated, and a postsalvage PET/CT scan gave her a Deauville score of 5. The patient was referred for CAR T-cell therapy and was placed on a waiting list. The patient opted to continue receiving treatment locally while waiting for availability at a cellular therapy center.
KHAN: Does anybody else have a different experience? Has anybody seen a 3- or 4-month lag [when looking to use CAR T-cell therapy] or any [have seen] astounding speed [in getting it], maybe even within a month?
BARSOUK: What’s the manufacturing time of the product? In your experience, what is the variation?
KHAN: Well, 3 weeks is the soft standard. About 20 to 21 days is the time it takes, depending on how close the manufacturing center is to you and what the shipping time is. Typically, it takes about 3 weeks for the whole manufacturing process to take place.
FAROUN: The question that we have to ask ourselves and to ask you, as an expert in lymphoma, is what kind of bridging therapy do you use for patients who are candidates for CAR T-cell therapy?
KHAN: I typically have used either GemOx or single-agent polatuzumab, which is common. If you go to any center, these are the 2 most common regimens that people use.
DISCUSSION QUESTIONS
- Who is involved in the decision-making for treatment?
- What are the key factors that influence your decision-making for third-line therapy? Change in mechanism of action
- National Comprehensive Cancer Network (NCCN) recommendation
- Risk factors
- Prior therapy/response to prior therapy
- Chance for long-term response
- Safety/tolerability
- Comorbidities
- Patient preference
- Convenience/logistics/schedule
- Performance status
- Other
KHAN: The first question is important: Who is involved in the decision-making? Is it you recommending it? Do you work with a CAR T-cell center to make that call about what to give? Do you discuss it with the patient? Do logistics come into play?
SANDHU: Typically, after the first-line therapy, [we] refer these patients out to our main site for specialists, and we try to get that available for the patient locally. Initially, with the first relapse or refractory disease or a relapse within 12 months, I typically end up referring the [patient] for CAR T-cell therapy.
KHAN: And the treatment that you would give before CAR T-cell therapy, or for bridging, if you are helping locally, do you just take their recommendations in what you would use?
SANDHU: Yes, I typically take their recommendations and we usually end up using GemOx.
KHAN: That’s how we work, too, and that is the most common. Dr Faroun, have you used tafasitamab before CAR T-cell therapy?
FAROUN: Yes, I have experience with tafasitamab. Again, it’s indicated for patients who are not candidates for transplant or who were not cleared for CAR T-cell therapy later, but I think tafasitamab is a good regimen. However, the dose of lenalidomide is, in my opinion, prohibitive.
A lot of patients will end up with pancytopenia with 20 mg oral daily, 3 weeks on, 1 week off. So I back off, especially if the patient is old [or has some renal failure] and usually I start with 15 mg, or down to 10 mg, but I have never had any luck with 20 mg.
KHAN: Yes, you’re right. The starting dose was 25 mg in the trial, and almost two-thirds of the patients required a dose reduction.1 So you’re right: Especially in the beginning, when the tafasitamab is on the weekly schedule, it’s a little tough to tolerate everything.
Has anybody used polatuzumab, or polatuzumab plus bendamustine and rituximab [pola + BR], in that pre–CAR T stage, or for bridging, for example? Probably not. I’ve used polatuzumab somewhat, too, and we talk about all these issues, but generally, we go by the NCCN guidelines.2
I think everybody takes patient preference and comorbidities into account. Has anybody come across a patient whom you’ve recommended for CAR T-cell therapy but, for whatever reason, they haven’t made it, or they don’t want to go, and [you] just do something different?
VARADI: Yes. I had a patient who was referred and the patient and the patient’s family declined.
KHAN: What was that for? Was it because it was far, or they just didn’t like the idea about the toxicity maybe? Or [something else]?
VARADI: I think it was mainly social issues.
KHAN: Do you remember what you chose to do with that patient, then?
VARADI: It’s actually ongoing. He is getting rituximab plus GemOx and we are discussing with them, again, the CAR T-cell therapy option or maybe autologous bone marrow transplant.
KHAN: Got it, so trying to convince them still.
VARADI: But I can’t force it.
KHAN: Yes, as it is with everyone. At least there are options now to keep things at bay until they can make that final decision.
CASE UPDATE
The patient received pola + BR.
DISCUSSION QUESTION
- Have you used pola + BR in any setting?
KHAN: Can anyone share [their] experience using [pola + BR]? Did the patient respond? Any toxicity issues? And I mean in any setting. It doesn’t have to be in a pre–CAR T setting.
FAROUN: I have used it, again, according to the guidelines, in a third-line setting. So the only thing I see a lot with this regimen is neuropathy and the need for dose reduction. If the patient is a candidate for CAR T-cell therapy, I would not use this regimen with bendamustine; I use it [only] with rituximab.
KHAN: That’s how most of us would do it, too. For those of you who have used it, and for those of you who haven’t used it, do you have any reaction to these updated data [NCT02257567]?3
What stood out to you? Are there any surprising data that you saw in this? Have you had similar experiences, as far as the responses are concerned?
COSTELLO: In this relatively highly pretreated group, which had a fair number of comorbidities and they weren’t candidates for [autologous stem cell transplant] or [CAR T-cell] therapy, the response rates were pretty impressive, especially that CR rate [42.5%].3 I wish it were a more durable CR, but in this patient population, this is still an impressive number.
KHAN: Have you used pola + BR, by any chance?
COSTELLO: I have not yet, and many times, as other people have commented, as patients get into the second-line setting, we refer these patients to a tertiary center to consider CAR T-cell therapy, and these sorts of medicines often get treated, still, down at the tertiary center.
KHAN: Is there anybody else who has had a different experience or who was surprised by these data, or who has any other thoughts?
SAMHOURI: I like that high-risk patients are represented in the trials. We have a good number of patients with activated B-cell subtype, approximately half of them. We have approximately 80% with primary refractory disease, or at least who relapsed quickly, within 12 months.3 Those are the patients that are difficult to treat and putting the response rate and the long follow-up in context, it gives us a good option for treatment for patients, at least those who are not going for transplant or CAR T-cell therapy.
KHAN: Is there anybody who thinks it’s a great advantage to have just 6 cycles, not something that you have to continue for too long?
FAROUN: Yes, that’s an advantage, 6 cycles and you stop, but the question is, to be honest with you, I am not that impressed with these data. The progression-free survival is only 9 months.3 I think we have better agents at this time, but not when the study was conducted. CAR T-cell therapy would be much better, in my opinion, and even tafasitamab is much better as a second-line therapy. I had some questions from the insurance company if the patient has CD79 [expression] on the malignant cells.
KHAN: Yes, they shouldn’t [ask you], because CD79 is naturally expressed in everyone. So different experiences, and you’ve had a good experience with tafasitamab plus lenalidomide.
FARON: My question then is, is CD79 100% overexpressed on diffuse bulky cells?
KHAN: Yes, typically it’s like CD20, and most of the patients will have it expressed. [It is one of the B-cell markers.]
DISCUSSION QUESTIONS
- How do you manage toxicities associated with pola + BR? Thrombocytopenia, neutropenia (dose reduction)?
- Peripheral neuropathy (hold to grade 1, then restart at lower dose)?
KHAN: How do you manage [the adverse events (AEs)]? How do you manage cytopenias? How do you manage the peripheral neuropathy [From the Data3]?
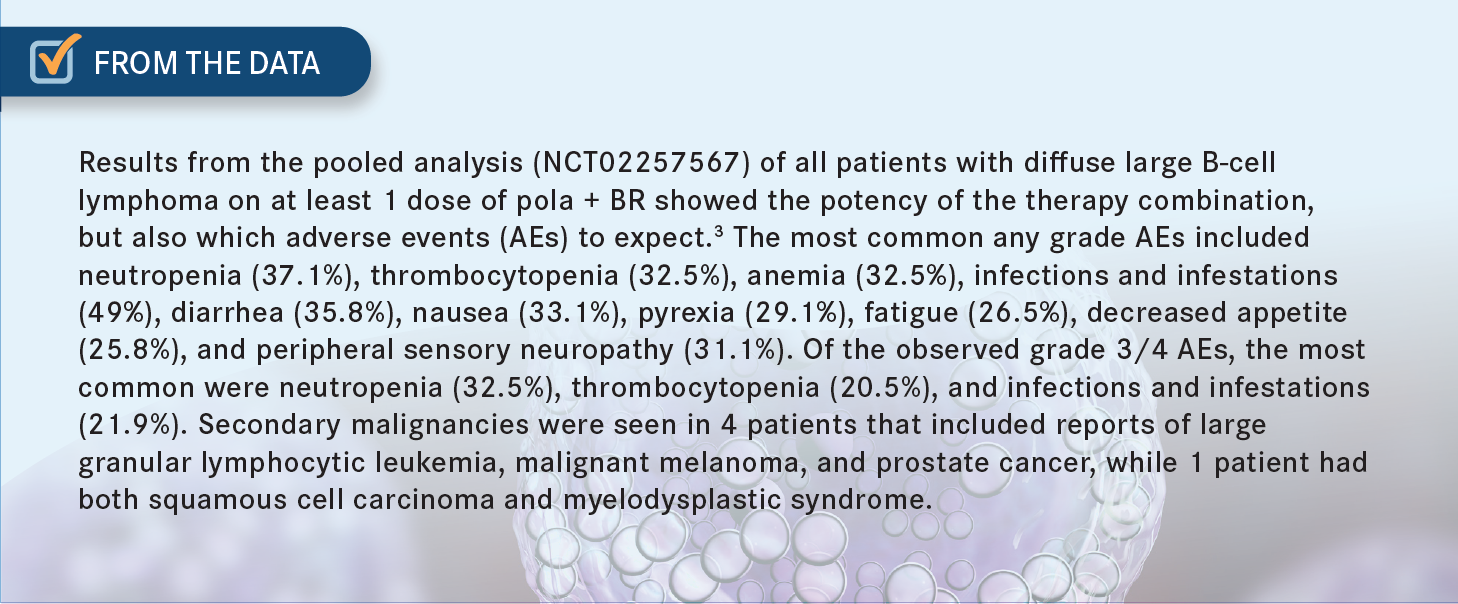
BARSOUK: I haven’t used it, but I would do exactly what is [suggested] here. For significant thrombocytopenia or neutropenia, I would hold until [the patient] recovers to a certain level of platelets and then implement dose reduction. And the same goes for peripheral neuropathy.
KHAN: I follow some of these protocols and work with pharmacists and advanced practice providers, and they make sure we are keeping a closer eye on the labs, and everything, and treat them appropriately. But, yes, that’s typically how we would follow these.
REFERENCES
1. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
2. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 2.2023. Accessed March 5, 2023. https://bit.ly/3YFDw8W
3. Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533-543. doi:10.1182/bloodadvances.2021005794