Roundtable Discussion: Mikhael Debates How the Addition of Frontline Daratumumab Affects Subsequent Therapy Use in Multiple Myeloma
Joseph Mikhael, MD, debated with a group pf peers about the impact of using frontline daratumumab on subsequent therapy.
Joseph Mikhael, MD

Joseph Mikhael, MD, professor of Applied Cancer in the Research and Drug Discovery Division of the Translational Genomics Research Institute of City of Hope Cancer Center, debated with a group pf peers about the impact of using frontline daratumumab on subsequent therapy.

ORLOWSKI: Normal fluorescence in situ hybridization [FISH] results are encouraging and take a patient out of the Revised International Staging System [R-ISS] stage III category, which is the worst group. If the β₂-microglobulin was elevated a little bit, that would put a person into the R-ISS stage II category, which gives them slightly increased risk—still a good prognosis overall, with induction therapy possibly to be followed by stem cell transplant. I think the only other assays that you could consider doing would be gene expression profiling—which, unfortunately, isn’t readily available and various sequencing panels...look for RAS or RAF mutations, but that also wouldn’t necessarily put them into a high-risk group. So, in general, such a patient would have a pretty decent prognosis.
MIKHAEL: When you think about ballpark prognosis of the patient, are you telling a patient [like that] 3 years, 5 years, 10 years? I’d say that we would expect survival of probably a decade or so, based on [a lower-risk level]. We’ve made tremendous advances in multiple myeloma, doubling—if not tripling—average survival in the past 15 years. But we’ve not had nearly as [much progress] with those patients at highest risk, such as patients with TP53 deletion or with particular cytogenetic abnormalities: translocations t(4;14) and t(14;16) and changes in chromosome 1.
MIKHAEL: Is transplant something that you’re going to consider routinely? Is that still something we should be doing in multiple myeloma, or is that old school?
ORLOWSKI: Just to be provocative, for a relatively standard- risk patient, especially if they achieve a complete remission [CR] and are MRD [minimal residual disease] negative, I think it is reasonable to think about collecting stem cells and storing them for a later transplant and then just doing maintenance. If they are high risk or if they don’t achieve CR and MRD negativity, then I think I’m more likely to recommend transplant.
MIKHAEL: I agree with that approach. I think we do have some very compelling data demonstrating the benefit of transplant; the data also show that, in certain patients, transplant can be delayed until first relapse.1,2
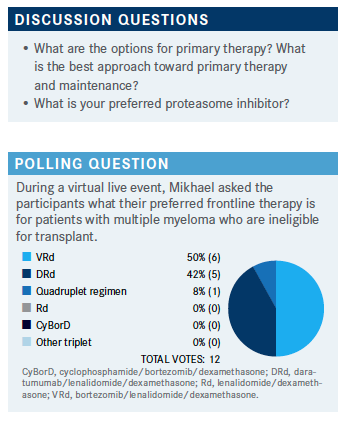
MIKHAEL: When you think about your options for primary therapy, it looks pretty convincing that you’re [all] at least going toward triplet therapy, which I think is the standard of care at this point, although there’s emerging evidence supporting quadruplets.3-5
MIKHAEL: Why VRd [bortezomib (Velcade), lenalidomide (Revlimid), dexamethasone] versus D-VRd [daratumumab (Darzalex)/VRd]? Why choose one over the other?
CHU: I think the first goal is to put a patient into CR as quickly and as easily as possible. I would save daratumumab for later use if a patient is a transplant candidate.
REDDY: I voted for D-VRd, but I haven’t actually used D-VRd. I’ve been using VRd with a very high success rate. If [we were to use] D-VRd, we don’t know what the second-line or third-line [therapy] would be. That is my biggest concern with using D-VRd, because [the data] are not airtight. I don’t think there are solid data [regarding] what to do after daratumumab. Can we use elotuzumab [Empliciti]? Carfilzomib [Kyprolis]?
MIKHAEL: That’s a fantastic point. The short way I would answer it is to say that I believe, as Dr Chu mentioned, in getting [the patient] into a deep response because depth of response in multiple myeloma correlates to duration. It’s clearly an important phenomenon. I think the community is tilted a little more toward more aggressive first-line therapy for multiple reasons: 1, because we’ve seen how these combinations have overcome the disease better; 2, we know that the first 2 remissions tend to be the longest remissions that a patient will experience; 3, every time we’ve worried about the so-called progression-free survival 2 [PFS2]—that is to say, how the patient will do after this therapy, whether it was on maintenance therapy or other induction strategies—we keep finding that the PFS2 is not compromised when you’ve given patients the best therapy that you have. This is because we have other [treatment] options and also because [giving people the best first-line therapy] gives people the best opportunity for a long-term remission. I made a comment at ASH [American Society of Hematology 2020 meeting]: “Saving the best for last is great for a Hallmark movie but not great for multiple myeloma.”
We have wonderful options, and I’ll mention those briefly. We look to the future of CAR [chimeric antigen receptor] T-cell therapy and bispecific therapy. Recently we had yet another drug approved for multiple myeloma: melphalan flufenamide [Melflufen],6 which is a different formulation of melphalan. We have a lot more options than we have had before. [Let’s consider] where daratumumab may be of value, in addition to VRd.
WANG: I think it all depends on the molecular profile of the patient. If the patient is young, I’d consider transplant.If the patient has standard-risk or low-risk disease, then I probably would choose VRd. But for someone who has a high-risk molecular profile, I really want to [get the patient into] a quick, deep response and then consider transplant. [In that case] I think D-VRd would be my choice.
MIKHAEL: I think that’s very rational until we have more data to guide us. We have very good evidence for VRd. We also have emerging evidence for D-VRd, but the data aren’t mature enough to distinguish high-risk disease from standard-risk disease if D-VRd is superior in [high-risk disease].4,5
MIKHAEL: Was anyone convinced by the FORTE study [NCT02203643] that we should use carfilzomib instead of bortezomib?1 People tend to think of carfilzomib for higher-risk disease. In what context are you using carfilzomib in frontline therapy?
WANG: I have not used carfilzomib in the front line. If someone had really high-risk disease, then I might consider it for frontline therapy. I feel like I still want to keep carfilzomib as my backup. I like to choose bortezomib for a couple of reasons. It’s very convenient to administer subcutaneously, and [later in treatment] you can change [the schedule] to once a week instead of twice a week. So I generally do not give carfilzomib at the front line.
AGARWAL: Same here. I don’t use carfilzomib as frontline therapy. Most of the insurance companies deny it. Bortezomib [can now be administered] subcutaneously. Most of the time I use it weekly, and the patents do just fine. That is why I’m not using carfilzomib [as frontline therapy].
MIKHAEL: I almost exclusively use bortezomib subcutaneously and weekly. Almost never will I give it twice weekly, with the possible exception of a patient who comes in with extremely high light chains and in renal failure.
I almost exclusively use carfilzomib weekly as well. It does help with convenience. For many of my patients, especially if they’re on carfilzomib long term and after they’ve achieved their maximal response, I just give it to them on day 1 and day 15, similarly to how bortezomib is sometimes administered.
REDDY: When you use bortezomib in the induction weekly, what is the most common schedule: 3 weeks on, 1 week off [or] 2 weeks on, 1 week off?
MIKHAEL: [That’s a great question because there are a lot of ways to give VRd.] I try to keep it simple. I almost always stick to a 4-week cycle, where I will give bortezomib weekly and I will give lenalidomide 21 days on, 7 days off. I’ll give the bortezomib subcutaneously, 1.3 mg/m2, and I’ll generally give full-dose lenalidomide at 25 mg, unless there’s a particular reason based on the patient profile to decrease the dose.
I tend to start with aggressive [treatment] and then drop the fourth week of bortezomib or reduce the lenalidomide dose [if necessary]. I find it much smoother and easier for patients to remember a 28-day cycle. I think we have some evidence that a fourth week of bortezomib helps. But if people use it 3 weeks out of 4 or use lenalidomide 2 weeks out of 4, I don’t think that’s unreasonable. I think it’s tricky to do 3-week cycling because then you have discordance between the lenalidomide and the bortezomib.

MIKHAEL: Do you only consolidate those patients that haven’t had a certain depth of response? Do you consolidate with more induction after a single transplant?
ORLOWSKI: Consolidation is something that I consider only if the patient has substantial disease burden after the first stem cell transplant; fortunately, that doesn’t happen very often. If they have a [heavy] disease burden, I think about a second transplant, assuming that I’ve seen some interval of benefit with the first one.
MIKHAEL: So you’re waiting to see that depth of response after a single transplant before you make the decision about consolidation?
ORLOWSKI: Correct. That’s 1 reason I [would choose] D-VRd as frontline therapy, even though it’s not yet FDA approved. The rationale is that you’re going to achieve your best response more quickly, and that may mean fewer cycles of induction with less toxicity, such as bortezomib- mediated neurotoxicity. [Regarding the] concern about daratumumab resistance and not being able to use it in second-line therapy, I would do D-VRd until maximum response, then collect stem cells and store them, and then put the person just on lenalidomide for maintenance. Then, if they were to progress, you could still go back and do daratumumab/pomalidomide [Pomalyst], daratumumab/carfilzomib, or [another] combination as second-line therapy.
MIKHAEL: That’s interesting. That’s why I made the point about maintenance. The [original] GRIFFIN results [NCT02874742]4,5 are a little different from those coming with the phase 3 study, regarding maintenance. In GRIFFIN, people were given daratumumab plus lenalidomide for 2 years, and then the daratumumab was stopped. Chances are, most of the patients are going to still be in remission at the end of those [first] 2 years because the median [time to remission] was 2 years [when patients were treated] with lenalidomide alone.
[When daratumumab is used with lenalidomide], I suspect that the majority of patients in GRIFFIN will not be having their first relapse [while] on daratumumab, so they may be able to have daratumumab [for second-line therapy] or maybe even isatuximab [Sarclisa] if you want to use a different CD38[-targeting therapy]. I know this PFS2 question is always a significant one and always makes me a little nervous. But the more I see the data and [firsthand] clinical results, the more I want to get people as quickly as possible into that deep remission.
WANG: I have a reservation about maintenance with daratumumab/lenalidomide. No. 1, it’s a lot more cumbersome [to administer an intravenous drug] for up to 2 years. In the community setting, it’s hard [to give], especially for older folks. No. 2, I just think: What’s the cost to the patient of getting such aggressive therapy for [that length of time]?
CHALLAGALLA: I agree with that. To convince patients to come from a hundred miles away for 2 years…there’s a lot of treatment fatigue and financial [expense].
MIKHAEL: I agree with you. To me, the proof has to be convincing, at least in standard-risk multiple myeloma, to change from lenalidomide alone. The preliminary data don’t convince me. They give me an indication. Frankly, the people in whom I’m using more than lenalidomide alone for maintenance are the high-risk patients, and I’m typically not giving them daratumumab. I’m giving them bortezomib, occasionally carfilzomib, if they’ve had significant neuropathy or something like that, even occasionally ixazomib [Ninlaro].
Because I think that the evidence we have is that lenalidomide maintenance improves both progression-free and overall survival compared with placebo, [though] maybe it doesn’t have the prolific impact we would want in high-risk disease.7 I could see that someone may want to use daratumumab/lenalidomide in a high-risk patient, but to use it [in standard-risk patients], especially considering the amount of time someone’s going to be on it, I think we need really convincing evidence.
Just in wrapping up, I would say that we don’t have perfect answers. I polled the International Myeloma Working Group recently and there was consensus that we’re likely moving toward quadruplet [therapy], including D-VRd, isatuximab and VRd, isatuximab and KRd, and daratumumab and KRd. [One exception is] elotuzumab and VRd, which did not have benefit over VRd in high-risk patients.
References:
1. Gay F, Cerrato C, Petrucci MT, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: results from the FORTE trial. J Clin Oncol. 2019;37 (suppl 15):8002. doi:10.1200/JCO.2019.37.15_suppl.8002
2. Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311-1320. doi:10.1056/NEJMoa1611750
3. Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29-38. doi:10.1016/S0140-6736(19)31240-1. Correction published online June 14, 2019.
4. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936-945. doi:10.1182/ blood.2020005288
5. Voorhees PM, Rodriguez C, Reeves B, et al. Daratumumab plus RVd for newly diagnosed multiple myeloma: final analysis of the safety run-in cohort of GRIFFIN. Blood Adv. 2021;5(4):1092-1096. doi:10.1182/bloodadvances.2020003642
6. FDA grants accelerated approval to melphalan flufenamide for relapsed or refractory multiple myeloma. FDA. Updated March 1, 2021. Accessed May 17, 2021. https://bit.ly/33OqonZ
7. Jackson GH, Davies FE, Pawlyn C, et al; the UK NCRI Haemato-oncology Clinical Studies Group. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, openlabel, randomised, phase 3 trial. Lancet Oncol. 2019;20(1):57-73. doi:10.1016/S1470-2045(18)30687-9

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More