Roundtable Discussion: Durvalumab Regimen Shows Efficacy in NSCLC
The PACIFIC study included patients with good performance status and unresectable, stage III NSCLC who did not progress after definitive platinum-based chemotherapy with concurrent radiation.
Mark A. Socinski, MD

During a Targeted Oncology Case-Based Roundtable event, Mark A. Socinski, MD discussed treatment of non–small cell lung cancer based on the PACIFIC trial.
Targeted OncologyTM: How do the results of the PACIFIC trial (NCT02125461) inform decisions about using durvalumab (Imfinzi) following completion of chemoradiotherapy for patients with stage III non–small cell lung cancer (NSCLC)?
SOCINSKI: The PACIFIC study included patients with good performance status and unresectable, stage III NSCLC who did not progress after definitive platinum-based chemotherapy with concurrent radiation.1 The patients had to be randomized within 6 weeks [of completion of chemoradiotherapy]. Patients were randomized to either the placebo control arm, which was the standard of care, or durvalumab for 1 year. The coprimary end points were progression-free survival [PFS] and overall survival [OS].
The PFS data, updated to approximately 3 years, show a hazard ratio [HR] of 0.51 [95% CI, 0.41-0.63] and pretty good separation of the Kaplan-Meier curves [median PFS, 17.2 vs 5.6 months].2 The 4-year update is pretty consistent [stratified HR, 0.55; 95% CI, 0.44-0.67; median PFS, 17.2 vs 5.6 months].3
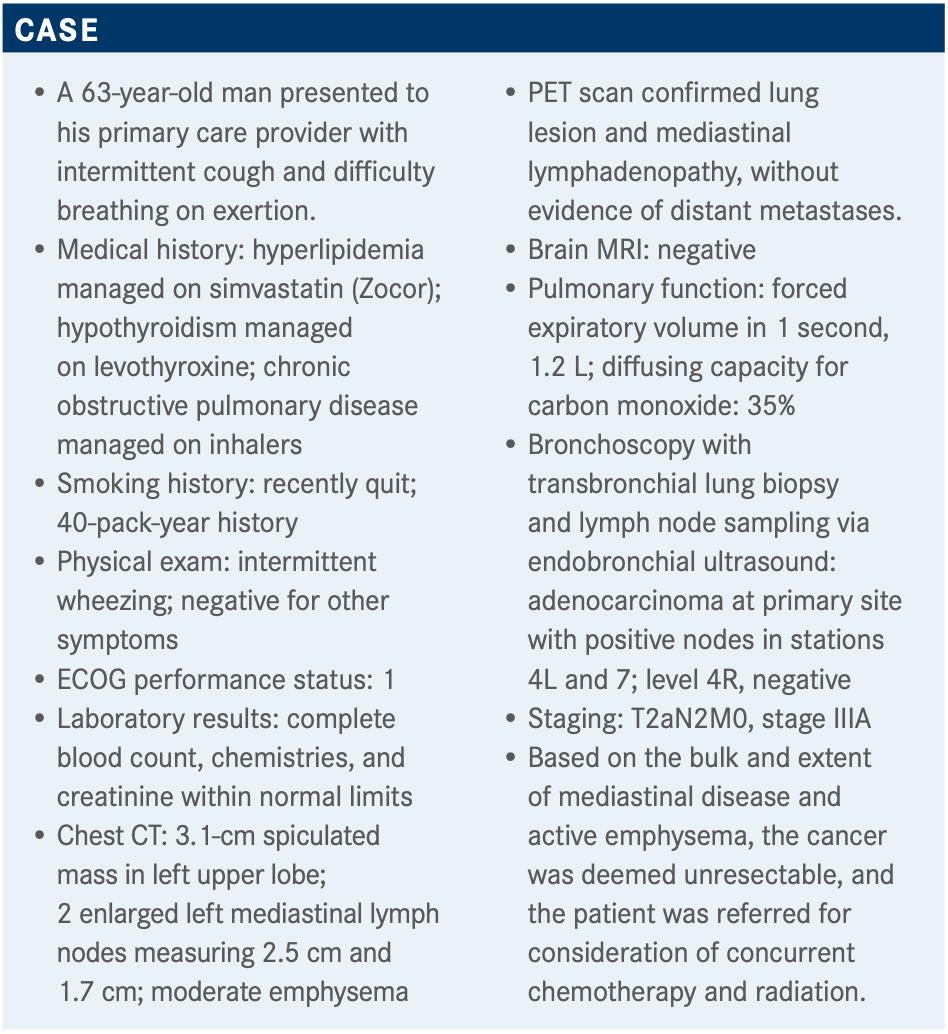
What was the initiation time of durvalumab on the PACIFIC trial?
In the original design of this trial, patients had to be randomized within 2 weeks [of completing chemoradiotherapy, but] that was problematic and accrual was slow. So, they amended the trial to bump it out to 6 weeks. The patients who started within 14 days had a better HR for PFS [unstratified HR, 0.39; 95% CI, 0.26-0.58] than patients who started after 14 days [unstratified HR, 0.63, 95% CI, 0.49-0.80].4 This begs the question: Is it just that [these patients were in better condition and therefore could] start early? Or is there something magic about giving anti–PD-L1 therapy so quickly after radiation? People who are much better immunologists than I am have an argument that earlier is better from the immune system’s point of view. I can’t explain that because I am not a good enough immunologist. That was the reason why they originally wanted to give [durvalumab] early, but they had to change it to 6 weeks because of feasibility issues.
The updated survival [data], presented at the 2020 European Society for Medical Oncology meeting, show that the 4-year OS curves are holding pretty steady with an HR of 0.71 [95% CI, 0.57-0.88].3 It took a long time for the median survival on the durvalumab arm to be reached, but it finally was reached at almost 4 years: 47.5 months [vs 29.1 months for placebo]. This is after chemoradiotherapy...it takes 3 months to do chemoradiotherapy, and then it might be 6 weeks from the completion of chemoradiotherapy to randomization and [initiation of] durvalumab. The control arm did pretty [well], but the durvalumab arm did much better. [It’s] nice to see this consistent benefit out to 4 years now.
Do EGFR and PD-L1 status influence your decision to recommend durvalumab?
The HRs for OS and PFS by subgroup all favor durvalumab [except for the EGFR-positive subgroup].3 There was a very tiny number of patients with the EGFR mutation [43 patients], so the confidence intervals [are very large]. So, I don’t know. Many of my colleagues are not giving consolidation durvalumab to patients with the EGFR mutation. They have 2 concerns: “Will it benefit the patient?” and “If they relapse and they’ve had an anti–PD-L1 agent, is it safe to give osimertinib [Tagrisso] after an immuno-oncology combination because of the risk of pneumonitis?"
PD-L1 testing was not required in the PACIFIC trial, and PD-L1 status [was unknown for] 37% of the patients. The prespecified analysis [with a] 25% cut point [shows that patients whose tumors] were strongly PD-L1 positive had a greater benefit.
The United States label [for durvalumab] is agnostic to PD-L1 status. However, the European authorities asked them to get a bit more granular [in] post hoc analysis. Why AstraZeneca agreed to do this, I don’t know, because this is not protected by the power of either stratification or randomization. And 37% of patients had an unknown status. The small number of patients wasn’t powered to address this issue. But in the less than 1% subgroup, the PD-L1–negative group, so to speak, there is no suggestion of a benefit. So, interestingly, based on this unplanned, unprotected, retrospective subset analysis, in Europe if a patient’s cancer is PD-L1 negative, they can’t get durvalumab paid for. I think these data are hypothesis generating; I don’t think they are standard-of-care setting. But that’s my personal view.
Are you concerned about the risk of pneumonitis with durvalumab?
There was concern about giving an agent that has a risk of pneumonitis—that being a PD-L1 inhibitor, [which] following radiation has a risk of pneumonitis. But [data from the PACIFIC trial showed that] grade 3/4 pneumonitis was 3.4% [for durvalumab vs 2.6% for placebo].4 There was a slight increase in any-grade pneumonitis with durvalumab [33.9% vs 24.8%], but it was mostly grade 1 or 2.4
How are you using induction therapy and consolidation therapy?
Many of us were doing a couple cycles of chemotherapy after chemoradiotherapy, and consolidation therapy now should be durvalumab. In the PACIFIC trial, 26.8% of the patients had induction therapy,4 and I have a bias [toward] induction therapy. I do it; we do it at our center. Our radiation oncologists like it because it buys them 6 weeks to get the radiotherapy organized and planned. Invariably there is some degree of shrinkage, so the tumor volume is a little less, and to me that is a good reason to do it. We give 2 cycles of induction therapy. Rather than give it as consolidation, I get my 4 cycles in [by giving] 2 cycles before chemoradiotherapy. Then I consider the 6 weeks of weekly carboplatin/paclitaxel as the equivalent of another couple cycles.
The National Comprehensive Cancer Network deleted the recommendation for consolidation therapy.5 If, for some reason, the patient has a contraindication to immunotherapy, then giving a couple cycles of consolidation or induction therapy is reasonable.
How would you manage pneumonitis in this patient?
Julie Renee Brahmer, MD, published guidelines for the management of toxicities of immunotherapies.6 It’s a nice paper to be familiar with and can be helpful to guide [treatment for] patients who do have pneumonitis.
Do you have any other thoughts on the treatment of patients on durvalumab?
Most of my patients get through a full year [of durvalumab therapy]. The most common reason I stop it during the year is because of an adverse event. The FDA has given that OK to move ahead with every 4 weeks, and that provides a lot more convenience.7
REFERENCES
1. Paz-Ares L, Villegas A, Daniel D, et al. PACIFIC: a double-blind, placebo-controlled phase III study of durvalumab after chemoradiation therapy in patients with stage III, locally advanced, unresectable NSCLC. Ann Oncol.2017;28(suppl 5):v605-v649. doi:10.1093/annonc/mdx440
2. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. doi:10.1056/NEJMoa1809697
3. Faivre-Finn C, Vicente D, Kurata T, et al. Durvalumab after chemo-radiotherapy in stage III NSCLC: 4-year survival update from the phase III PACIFIC trial. Ann Oncol. 2020;31(suppl 4):S1178-S1179. doi:10.1016/j.annonc.2020.08.2281
4. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med.2017;377(20):1919-1929. doi:10.1056/NEJMoa1709937
5. NCCN. Clinical Practice Guidelines in Oncology. Non-small cell lung cancer, version 8.2020. Accessed April 29, 2021. https://bit.ly/30sRU9e
6. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714-1768. doi:10.1200/JCO.2017.77.6385
7. IMFINZI (durvalumab) approved in the US for less-frequent, fixed-dose use. News release. AstraZeneca. November 20, 2020. Accessed April 29, 2021. https://bwnews.pr/398dmFs
