Lenvatinib/Everolimus Shows Potential as Second-Line Therapy After CheckMate 9ER Regimen in RCC
The CheckMate 9ER study compared nivolumab plus cabozantinib with sunitinib and found that as disease becomes less favorable by prognostic risk. Promise has now been shown for this population with the combination of lenvatinib and everolimus.
Robert A. Figlin, MD

During a virtual Targeted Oncology Case-Based Roundtable event, Robert A. Figlin, MD discussed frontline therapy for renal cell carcinoma (RCC).
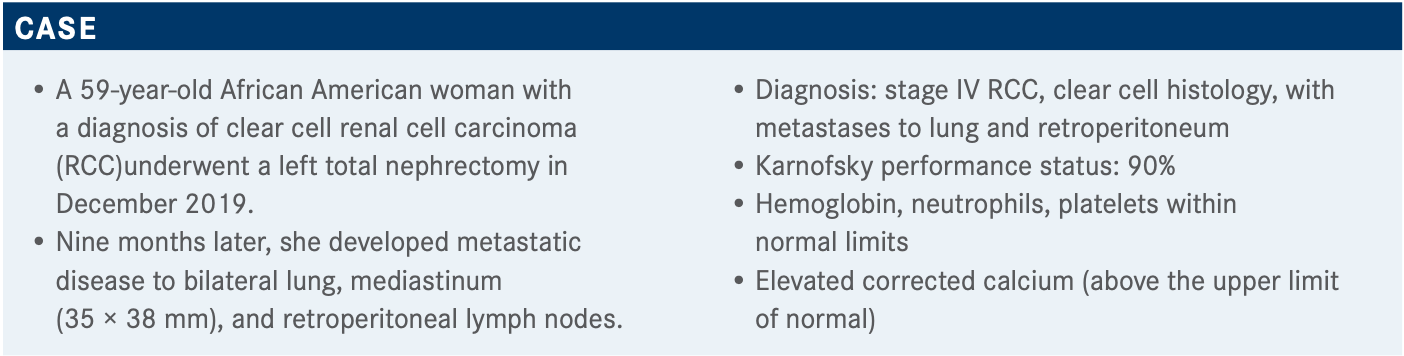
Targeted OncologyTM: What frontline therapy are you most likely to choose for this patient who has stage IV clear cell RCC with a poor or intermediate risk?
FIGLIN: The National Comprehensive Cancer Network [NCCN] guidelines that came out earlier in 2021 recommend 2 [combination regimens] that are category 1: axitinib [Inlyta] plus pembrolizumab [Keytruda], or ipilimumab [Yervoy] plus nivolumab [Opdivo].1 A lot of that [choice] is based upon a person’s [particular] comfort level. Cabozantinib [Cabometyx] plus nivolumabis also [an option], but immunotherapy [IO] plus tyrosine kinase inhibitor [TKI] or IO plus IO are the approaches [we use].
Can you describe some of the current targeted therapy approaches in RCC?
We now know that there’s a large rationale for VEGF TKIs because of loss of VHL function, and clear cell RCC, which this patient had, generates VEGF.2 But there’s also evidence that an mTOR can become active in this population. We now know that some of the resistance pathways to VEGF are through MET and [FGF]. We now have agents like cabozantinib that hit MET as well as VEGF. Lenvatinib [Lenvima] hits FGF as well as VEGF. We have a variety of single agents. I think one of the remarkable things about kidney cancer is we know it is an immunologically responsive disease.3-5 But it’s not an immunologically responsive disease because of a large number of somatic mutations.3 It’s for other reasons that the kidney cancer responds to IO therapy.
Which data influence your choice of frontline treatment in those with poor or intermediate risk?
When one considers the standard frontline treatments for intermediate and poor risk, the major studies looked at pembrolizumab/axitinib, avelumab/axitinib, ipilimumab/nivolumab, cabozantinib, and cabozantinib/nivolumab.6-9 The HRs [for death] are somewhere between 0.53 and 0.80. The objective responses vary from about 20% with just the TKI all the way up to almost 60% with pembrolizumab/axitinib.6,7 The complete response rate with either IO-IO or IO-TKI is about 3% to 4% all the way up to 10% with ipilimumab/nivolumab.6-9
This is important because [you must] recognize that decision making in treating kidney cancer often depends upon your goals of therapy for the individual patient. Many people might argue that if this patient were symptomatic, because of their lively metastatic disease, they’d like to get a more rapid response, and so they might use an IO-TKI. [However, in] a person like this, who has asymptomatic metastatic disease, IO-IO would have sufficient time to demonstrate benefit.
Which data support the use of nivolumab and cabozantinib in a patient like this?
The [CheckMate 9ER study (NCT03141177) compared nivolumab plus cabozantinib with sunitinib (Sutent)] and found that as [the] disease becomes less favorable by prognostic risk, the benefits of IO-TKI with nivolumab/ cabozantinib are even better.8 [The combination showed consistent progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) benefits over sunitinib across key baseline characteristics, including International mRCC Database Consortium risk status, tumor PD-L1 expression, and bone metastases.] PD-L1 expression did not matter. Also, it’s important to recognize that, because of the history of cabozantinib’s benefit in patients with bone metastases, you can also see a benefit [with] nivolumab/cabozantinib. That would be an important consideration when thinking about how to choose regimens for specific subpopulations of patients.
OS was a secondary end point [in the CheckMate 9ER study].8 [Neither arm of the study reached the end point], but the HR was 0.6 [98.89% CI, 0.40-0.89; P = .0010]. With 2-year follow-up, the HR is now 0.66 [95% CI, 0.50- 0.87].9 I think that nivolumab/cabozantinib will have a survival benefit, although it’s a secondary end point.
[Blinded independent central review of] the ORR and best overall response [showed that] almost 60% [95% CI, 50.1%- 61.2%; P < .0001] of the patients had an objective response, and 8% had a complete response [CR].8 But what’s incredibly impressive is the progressive disease. Only 5.6% of patients had progressive disease [after] starting on this regimen. So, we can have a conversation with our patients to say, “You have more than a 90% chance that you’ll obtain either a CR, partial response, or no new or growing disease with the use of this therapy.” Time to response was relatively short, and durations of response were more than 18 months.
At the 23-month follow-up, the ORR was maintained at 54.8%.9 The CR rate was a little bit higher at 9.3%, and the disease control rate was just about 90%.
Which safety issues concern you most when prescribing nivolumab and cabozantinib?
Nivolumab is dosed at 240 mg intravenously every 2 weeks.10 Many physicians probably do [480 mg] every 4 weeks if you’re giving it in the clinic. Probably the important thing to recognize is that cabozantinib, when combined with nivolumab, is given at a dose of 40 mg [daily] rather than 60 mg [daily], which is the dose when it is given as monotherapy TKI.11 The 40-mg cabozantinib dose is very well tolerated. It’s different than giving 60 mg of cabozantinib as monotherapy. [In the CheckMate 9ER study], treatment-related adverse events [AEs] related to discontinuations at 2 years were about 10% for nivolumab, 7% for cabozantinib, and 6.6% for the combination.9 The [percentage of] grade 3 or higher treatment-related AEs was about the same for nivolumabplus cabozantinib and sunitinib for hypertension, handfoot syndrome, and diarrhea.8
What are potential subsequent or second-line therapies after VEGF TKI in intermediate/poor-risk metastatic clear cell RCC?
You have a variety of category 1 treatment choices; [the NCCN recommendations include axitinib, nivolumab, cabozantinib, and the combination of lenvatinib plus everolimus [Afinitor].1 The challenge with NCCN guidelines is that many of these category 1 options were approved prior to IO-TKI. So, [these options] stay in these recommendations, but using cabozantinib after nivolumab/cabozantinib makes no sense; using nivolumab after nivolumab/cabozantinib makes no sense; and not a lot of data support using nivolumab/ipilimumab after nivolumab/cabozantinib.
Lenvatinib plus everolimus was studied in a randomized phase 2 trial in patients with TKI-refractory [disease], 100% of whom had 1 prior therapy.12 About a [third] of the patients had good risk, a third intermediate risk, and a third poor risk. The ORR was quite impressive [at 43%]. The PFS was almost 15 months [HR, 0.40; 95% CI, 0.24-0.68; P = .0005], OS was more than 2 years [HR, 0.73; 95% CI, 0.57-0.93; P = .002]. Dose reductions, which are required with a lot of people with grade 3 and grade 4 toxicities, [occurred in 71% of patients]. Remember that the dose for lenvatinib/everolimus starts at 18 mg of lenvatinib and 5 mg–not 10 mg– of everolimus.13
How do you manage toxicities associated with TKI therapy–specifically, for lenvatinib plus everolimus?
I think the dose modification regimen for lenvatinib/everolimus is pretty simple. Unlike IO-TKIs or IO-IOs, where each of the agents can be contributing to some of the grade 3 and grade 4 toxicities, with lenvatinib/everolimus, you either have a VEGF toxicity or you have an mTOR toxicity.12,14 They are different enough that you might be able to pick out which of those to stop, dose-modify, restart. It’s [about] identifying the toxicity, managing the one that’s most related to the agent, and then restarting [the agent] at a reduced dose once [the toxicity has] resolved to grade 1 or less.
References:
1. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 4.2021. Accessed May 1, 2021. https://bit.ly/33kJ8er
2. Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. doi:10.1038/nrdp.2017.9
3. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500-2501. doi:10.1056/NEJMc1713444
4. Şenbabaoğlu Y, Gejman RS, Winer AG, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol. 2016;17(1):231. doi:10.1186/s13059-016-1092-z
5. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1-2):48-61. doi:10.1016/j.cell.2014.12.033
6. Escudier B. Combination therapy as first-line treatment in metastatic renalcell carcinoma. N Engl J Med. 2019;380(12):1176-1178. doi:10.1056/NEJMe1900887
7. Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer. 2018;94:115-125. doi:10.1016/j.ejca.2018.02.012.
8. Choueiri TK, Powles T, Burotto M, et al. Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: first results from the randomized phase III CheckM9. Motzer RJ, Choueiri TK, Powles T, et al. Nivolumab + cabozantinib (NIVO+CABO) versus sunitinib (SUN) for advanced renal cell carcinoma (aRCC): outcomes by sarcomatoid histology and updated trial results with extended follow-up of CheckMate 9ER. J Clin Oncol. 2021;39(suppl 6):308. doi:10.1200/JCO.2021.39.6_suppl.308
10. Opdivo. Prescribing information. Bristol Myers Squibb Co; 2021. Accessed May 1, 2021. https://bit.ly/3nUdSfZ
11. Cabometyx. Prescribing information. Exelixis; 2021. Accessed May 1, 2021. https://bit.ly/2Syxb2H
12. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473-1482. doi:10.1016/S1470-2045(15)00290-9
13. Lenvima. Prescribing information. Eisai; 2020. Accessed May 3, 2021. https://bit.ly/3eoCiLh
14. Motzer RJ, Escudier B, McDermott DF, et al; CheckMate 025 Investigators. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803-1813. doi:10.1056/NEJMoa1510665

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen