Durvalumab After Chemotherapy Helps Manage Toxicities in NSCLC Treatment
Chemotherapy for the treatment of non-small cell lung cancer can be quite toxic, however, durvalumab after chemotherapy can help manage toxicities while improving overall survival, according to the PACFIC trial.
Jamie E. Chaft, MD

During a Targeted Oncology Case-Based Roundtable event, Jamie E. Chaft, MD discusses durvalumab after chemotherapy in order to reduce toxicities in non-small cell lung cancer (NSCLC).
Targeted OncologyTM: What is your overall impression of the results of the PACIFIC trial?
CHAFT: The PACIFIC study [NCT02125461] was a randomized, phase 3 placebo-controlled trial designed in a way that strongly resembled the real-world setting.1 The trial took patients with unresectable locally advanced [non–small cell lung cancer (NSCLC)] who had not progressed during definitive chemoradiotherapy with a platinum-based doublet. The patients had to have had at least 2 cycles of platinum-based therapy. Patients had a good ECOG performance status, either 0 or 1, and a reasonable life expectancy, and they were randomized in a 2:1 fashion to durvalumab [Imfinzi] 10 mg/kg versus placebo every 2 weeks for up to a year. The 2 coprimary end points were PFS [progression-free survival] and OS [overall survival].
At the 4-year data update, the median PFS with durvalumab was 17.2 months and the placebo was 5.6 months.2 This is exceptionally sobering and shows that we have a lot of work to do. The hazard ratio [PFS, 0.55; OS, 0.71] and the median PFS and OS were exceptionally good for an unselected patient population.3
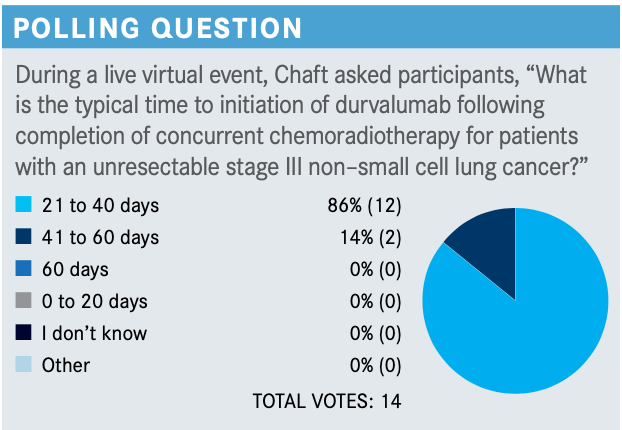
When was durvalumab given on the PACIFIC trial after chemoradiotherapy?
A subanalysis of the PACIFIC trial, specifically the hazard ratio of the forest plot results, showed a fairly striking benefit with the initiation of durvalumab within 14 days of radiotherapy [<14 days HR, 0.42; ≥14 days HR, 0.81].3
When the PACIFIC study was launched, it required initiation of durvalumab within 14 days, which was the reason [my organization] didn’t participate in the study. They quickly learned that initiation within 14 days is not feasible for most patients and the range was expanded to up to 42 days. But among patients who could start durvalumab within 14 days, an advantage was observed. [These data are from] a PFS subset, and there are probably many factors that go into this analysis, but these are the data.
Do you think the results support the use of durvalumab for the various subsets tested?
At the [trial’s] 4-year landmark, the OS was 50% versus 36%, and the confidence intervals do not overlap.2 We can confidently say that durvalumab improves OS. There is striking improvement in OS both in terms of hazard ratio and at each subsequent landmark.
[Looking at various subsets], the majority show an advantage for durvalumab versus placebo. However, the error bars suggest that there is a clear doubt regarding the EGFR-positive subset.
In my practice, we don’t get enough tissue for molecular testing in a stage III [tumor] if an endobronchial ultrasoundis done. But in this data set, the EGFR-positive population was questionable. For nearly every other group, durvalumab either trends or is statistically better than placebo.
Do the results justify testing for PD-L1 expression?
In the PACIFIC study, PD-L1 expression was defined as positive if it was greater than or equal to 25%. For the high PD-L1 expressers, there is a trend showing greater benefit [with treatment], and for all patients, including those with known and unknown PD-L1 status, there is an advantage in terms of OS and PFS.⁴ A post hoc analysis was requested by the European Medicines Agency for PD-L1–negative tumors [1% to < 25%]. Although there is a trend toward a PFS advantage, there is no OS advantage. In the United States, this was not considered a game changer, whereas in Europe, durvalumab is only approved for PD-L1–positive patients.
What do the results indicate in terms of safety?
In terms of toxicity, [durvalumab] was really well tolerated and there were no unexpected effects. There was a concern with pneumonitis, but the high-grade pneumonitis rates were not different between the placebo and the durvalumab arms. For all pneumonitis cases, the [incidence] was 34% versus 25%, whereas for grade 3 or 4 pneumonitis, there was less than a percentage point difference [between the 2 arms].4
What guidelines do you follow when deciding when to initiate treatment?
The National Comprehensive Cancer Network guidelines clearly indicate that durvalumab [should be given] every 2 weeks, which is consistent with the FDA label.5
Interestingly, it no longer recommends consolidation chemotherapy for patients who used to get low-dose weekly therapy and then 2 full-dose cycles, as that was not utilized in the PACIFIC study.5 ASCO [American Society of Clinical Oncology] has updated its guidelines because of the pandemic to a more liberal timeline for initiation of therapy. To minimize exposure, ASCO is recommending hypofractionated radiation [therapy] and greater dosing intervals, such as [every 4 weeks].6
What is your approach toward pneumonitis diagnosis and management for patients receiving a single-agent checkpoint inhibitor?
There are many algorithms for managing unrelated adverse events. We test for respiratory viruses and COVID-19, and if the infectious work-up is negative, [there is] a low threshold for steroids. For grade 1 [pneumonitis], we often watch and wait with close pulse oximetry monitoring. For grade 2, we hold [the immune checkpoint inhibitor] and initiate steroids with a slow taper of usually 4 to 6 weeks.7
For grades 3 and 4 [pneumonitis], we almost always permanently stop [the immune checkpoint inhibitor]. [We have a] very low threshold for adding steroid-sparing immunosuppression. Supplemental oxygen [is given] if needed. We often add empiric antibiotics to avoid grade 5 pneumonitis. Bronchoscopy is often offered to rule out infectious etiologies. Most patients with grade 3 or 4 [pneumonitis] rarely require hospitalization for pneumonitis management.7
REFERENCES
1. Paz-Ares L, Villegas A, Daniel D, et al. PACIFIC: A double-blind, placebo-controlled phase III study of durvalumab after chemoradiation therapy in patients with stage III, locally advanced, unresectable NSCLC. Ann Oncol. 2017;28(suppl 5):v634. doi:10.1093/annonc/mdx440.049
2. Faivre-Finn C, Vicente D, Kurata T, et al. LBA49 Durvalumab after chemoradiotherapy in stage III NSCLC: 4-year survival update from the phase III PACIFIC trial. Ann Oncol. 2020;31(suppl 4):S1178-S1179. doi:10.1016/j.annonc.2020.08.2281
3. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. doi:10.1056/NEJMoa1809697
4. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi:10.1056/NEJMoa1709937
5. NCCN. Clinical Practice Guidelines in Oncology. Non-small cell lung cancer, version 8.2020. Accessed May 4, 2021. https://bit.ly/30sRU9e
6. Singh AP, Berman AT, Marmarelis ME, et al. Management of lung cancer during the COVID-19 pandemic. JCO Oncol Pract. 2020;16(9):579-586. doi:10.1200/OP.20.00286
7. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714-1768. doi:10.1200/JCO.2017.77.6385

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More