PARTICIPANT LIST
Joyce Feagin, MD
Shan Guo, MD
Inna Shmerlin, MD
Katherine Wang, MD, PhD
Giancarlo Moscol, MD
Marnin Merrick, MD
Andrew Kovoor, MD
Henry Q. Xiong, MD, PhD
Narotham R. Thudi, MD
EVENT REGION Louisiana and Texas
During a Targeted Oncology™ Case-Based Roundtable™ event, Nizar M. Tannir, MD, discussed with participants their experiences with the combination of lenvatinib and pembrolizumab, including the dosing schedule of pembrolizumab and finding a tolerable dose of lenvatinib.
Nizar M. Tannir, MD (MODERATOR)
Professor
Ransom Horne Jr Professorship for Cancer Research
Department of Genitourinary
Medical Oncology
Division of Cancer Medicine
The University of Texas MD Anderson Cancer Center
Houston, TX

Joyce Feagin, MD
Shan Guo, MD
Inna Shmerlin, MD
Katherine Wang, MD, PhD
Giancarlo Moscol, MD
Marnin Merrick, MD
Andrew Kovoor, MD
Henry Q. Xiong, MD, PhD
Narotham R. Thudi, MD
EVENT REGION Louisiana and Texas
CASE SUMMARY
A 59-year-old African American woman with a left renal mass underwent left radical nephrectomy in December 2019, revealing clear cell renal cell carcinoma (RCC). Nine months later, she developed nodules in both lungs, with mediastinal (35 × 38 mm) and retroperitoneal lymph nodes. A lung biopsy confirmed stage IV RCC with clear cell histology. She had a Karnofsky performance status of 90%. Her hemoglobin level was 11.1 g/dL, and her corrected calcium, neutrophil, and platelet levels were within normal limits. The patient is scheduled to begin pembrolizumab (Keytruda) at 200 mg every 3 weeks plus lenvatinib (Lenvima) at 20 mg daily.
DISCUSSION QUESTIONS
TANNIR: Lenvatinib at 20 mg is [given as] 2 capsules of 10 mg each, and it’s taken orally daily at the same time.1 I think the interesting thing about this capsule is that it can be swallowed whole with or without food [or] dissolved in a glass of liquid if the patient can’t swallow the pill as a whole. The pembrolizumab is either 200 mg every 3 weeks or 400 mg every 6 weeks.2 We can choose how we give pembrolizumab—at what schedule and at what dose.
As per the protocol [of] the CLEAR study [NCT02811861], the starting dose of lenvatinib is 20 mg a day, and for toxicity management after interruption, you reduce the dose down to 14 mg [Figure 11].
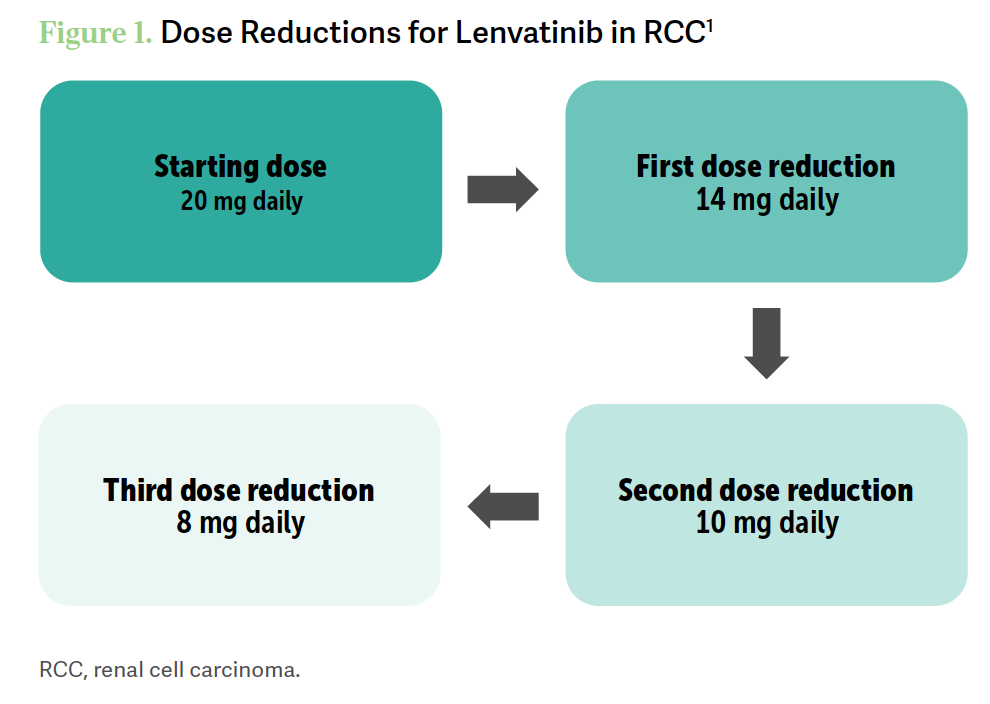
The second dose reduction is 10 mg, and then the third is 8 mg a day. You can withhold, dose reduce, or discontinue lenvatinib as recommended [and] withhold…and discontinue pembrolizumab as recommended too. Obviously, you don’t reduce the dose [of pembrolizumab].
For patients for whom you choose pembrolizumab/lenvatinib, what dosage administration schedule for pembrolizumab do you use more often? Is it the 3-week regimen, 200 mg or is it the 6-week regimen, 400 mg? Do you switch from one to another? What dose of lenvatinib do you usually start [at for] patients that you decide to treat with lenvatinib/ pembrolizumab?
FEAGIN: I have some experience with lenvatinib/pembrolizumab, and I use the 200-mg-every-3- weeks [pembrolizumab dose] because I see the patients fairly frequently. I have had some issues with starting the lenvatinib at 20 mg, so I usually start at 14 mg.
TANNIR: [Do] you start at 14 mg regardless of their age or their comorbidities?
FEAGIN: If [they are] younger, fit patients, I would start with 20 mg, but most of my patients are older and frailer.
TANNIR: If your patients are [older] and frail and have comorbid illnesses, I understand. But the starting dose in the CLEAR study was 20 mg a day, 2 capsules every day.3 A good percentage of patients had to interrupt therapy and dose reduce to 14 mg. Some reduced to lower [than 14 mg].
GUO: I have not used the 20-mg [dose] as first-line [therapy] because most of my patients are in the community [setting] and they’re older. Many of the patients have chronic kidney disease, so the maximum [dose] I have started with is 18 mg. The reason I do 18 mg is…because then [the patient] gets a whole bunch of 4-mg and 10-mg [capsules, which makes it] easier for me to use different dosages combined. If the patient has any adverse events [AEs], I can use the 10-mg or 4-mg [capsules]. The maximum I start with is 18 mg, but based on the patient’s tolerance I’ve used 12 mg, 14 mg, and 18 mg.
SHMERLIN: I tend to use 14 mg. From prior experience using lenvatinib as a single agent [for patients with] hepatocellular carcinoma, I don’t find that it’s as well tolerated in higher doses. We’re in the community [setting], [so] our patients are [older] and a little frailer. I’ve started a patient at 14 mg with the intention of going up but that never happened.
WANG: I usually start with 14 mg [for] pretty much everybody; even with the 14-mg dose, sometimes they still [need] to have dose modification with lenvatinib. Pembrolizumab, I usually do every 3 weeks, especially for those [frail] patients [because] you need to see them at least every 2 weeks or every 3 weeks.
MOSCOL: I agree with pembrolizumab every 3 weeks. You need to keep a close eye on these [therapies], especially because they tend to compound fatigue.
In my experience, that ends up being quite limiting and may end up forcing you to also cut back on their lenvatinib. I usually settle at 14 mg, and I can try 18 mg sometimes, but the great majority go to 14 mg.
DISCUSSION QUESTIONS
TANNIR: Some of you like to start at a lower dose, but [in the CLEAR trial] for all those patients who started at the full dose, 20 mg, around 69% of them required dose reduction of lenvatinib in the lenvatinib/pembrolizumab…arm of the study [Table1,2].
Table. Results from the CLEAR trial
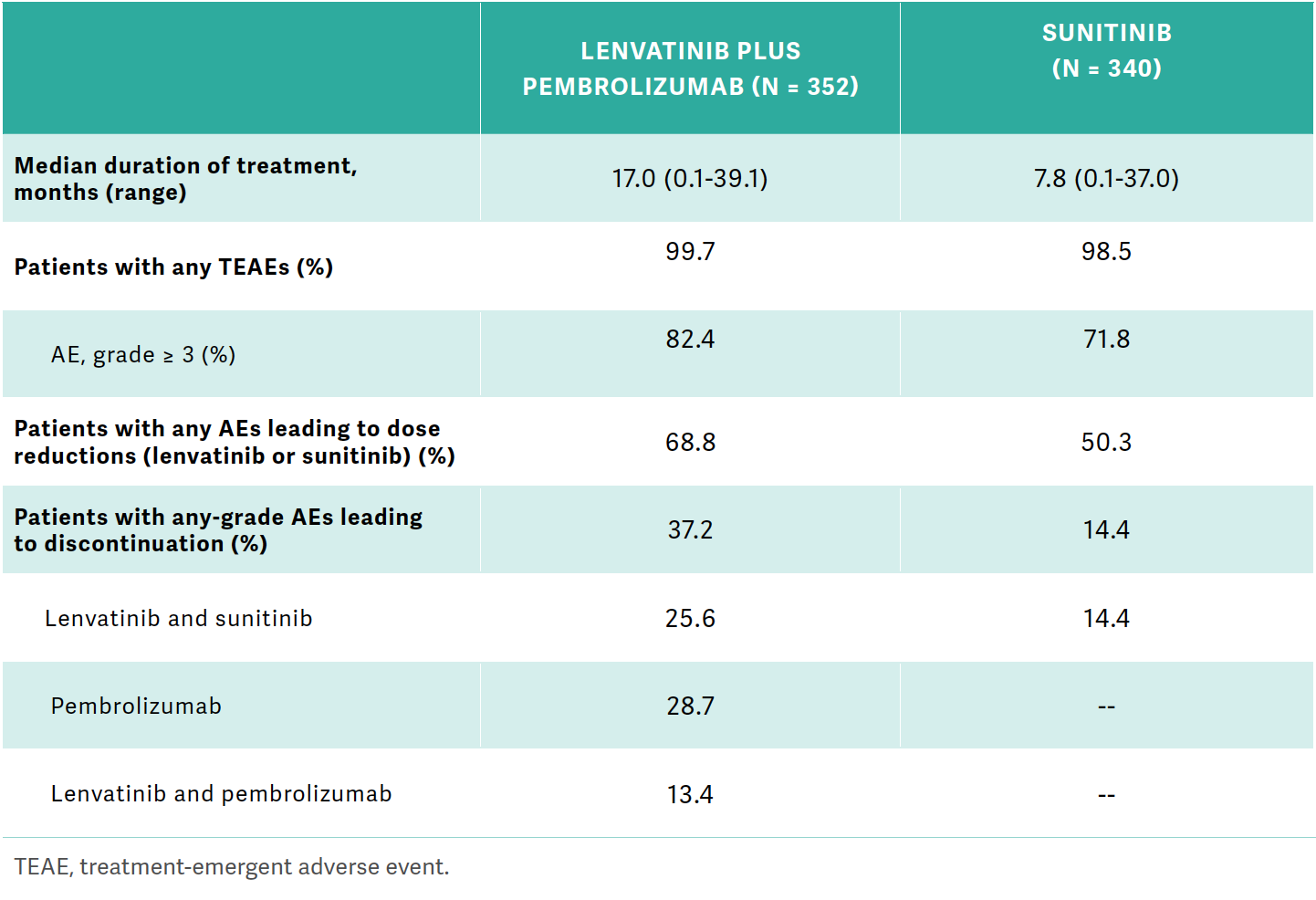
[There were dose] reductions in 50% of [patients in] the sunitinib [Sutent] control arm…. Any-grade AEs leading to discontinuation were 37% with lenvatinib/pembrolizumab…and 14% with sunitinib. For lenvatinib/pembrolizumab, 25.6% discontinued lenvatinib…, 28.7% discontinued pembrolizumab, and discontinuation of both lenvatinib and pembrolizumab [was] 13.4%.3
MERRICK: That trial was conducted using a dose of lenvatinib of 20 mg. [But] during the discussion with our colleagues…many use a lower dose. Do we know what type of doses the patients on the trial received, [whether] they had dose reductions, [and] the actual dose they were receiving?
TANNIR: You heard from your colleagues that the patients they see are frail, may be [older, or] they have comorbid illnesses.… What you do in your practices…is different than the patients who are recruited to the trials.
There are strict eligibility criteria to go on the trial. The sponsor of the trial and the investigators who participated in the trial wanted to start all the patients on the full dose of 20 mg a day.
As you saw, 69% of them had AEs that required interruption and dropping the dose. And that happened [for many at] 6 weeks, and some of them had that interruption later on.
I think the important thing from the study is that even if patients started with 20 mg and…required dose reduction to 14 mg—and even after that to 10 mg—some of the responses were maintained. My practice is, when I see a patient who is eligible or I believe will tolerate 20 mg and is best treated with that regimen [vs] other regimens, I start them at 20 mg lenvatinib to mirror the trial. Of course, we monitor them frequently, every 3 weeks, to make sure that they’re tolerating the treatment well. And if I see that after 6 weeks or at any time, even earlier at 3 weeks, they’re not tolerating 20 mg, we give them a break until the AEs improve to grade 1 or resolve and then reduce the dose down to 14 mg.
KOVOOR: I was impressed with the response rates. The AEs make me a little hesitant. I’d probably start them off on 14 mg just to prevent that dose reduction because my patients tend to be a little on the older side. I don’t think they would tolerate the 20 mg too well. If I had a young guy who was fit, I’d probably go with the 20 mg.
XIONG: First, these efficacy data are very impressive. You have a very high response rate and a pretty significant CR [complete response] rate [of] 17%.3 If you look at AEs, it is a concern to me [Figure 23]. The diarrhea rate is high. The discontinuation rate is 37%, so that’s significant.
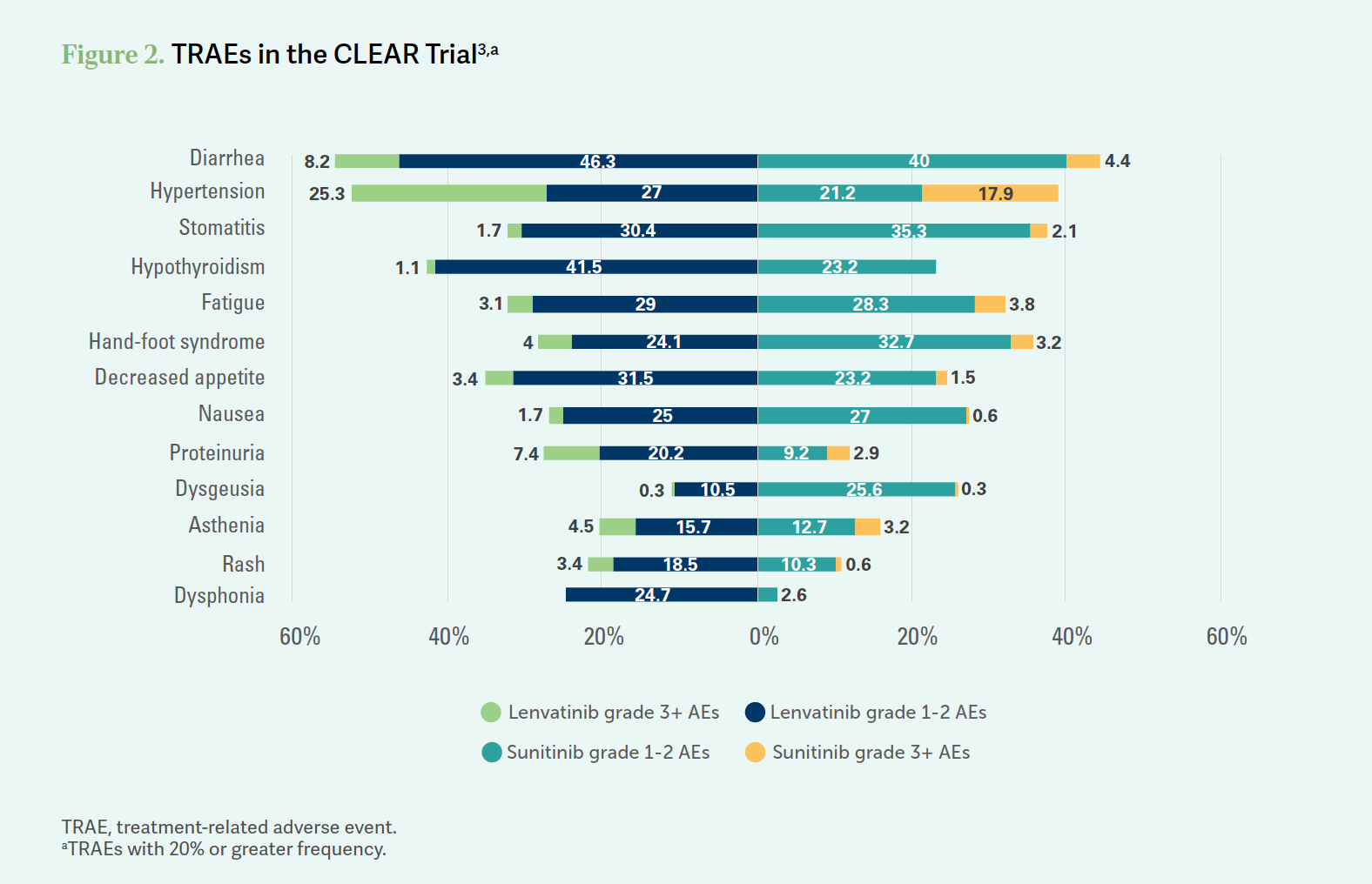
TANNIR: When we [look at] the other 2 immunotherapy plus tyrosine kinase inhibitor [TKI] regimens, the discontinuation rate is also in the same ballpark.3-5 I think these regimens can be tough, of course. When you look at nivolumab [Opdivo] plus ipilimumab [Yervoy], the discontinuation rate was 22%.6 It’s because [with] chronic administration of TKIs, regardless of what TKI you use, over the years these patients are going to eventually discontinue.
THUDI: The data are impressive, and I have [had] at least 6 patients in the past year on this regimen. I usually try and tend to start at 18 mg or 20 mg if they’re young and have good performance status. But…most of them settle down at 14 mg. For 2 of my patients, I had to reduce the dose because of significant blisters in the feet as well as in the hands. One of them had significant proteinuria, and one of them had extreme fatigue and hypertension problems. I’ve been scanning them, and they’re still responding…not just stable disease, so they’re continuing the therapy.
REFERENCES
1. Lenvima. Prescribing information. Eisai Inc; 2022. Accessed May 22, 2023. https://bit.ly/3olUCfN
2. Keytruda. Prescribing information. Merck Sharp & Dohme LLC; 2023. Accessed May 22, 2023. https://bit.ly/3xdYO1X
3. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
4. Rini BI, Plimack ER, Stus V, et al; KEYNOTE-426 Investigators. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi:10.1056/NEJMoa1816714
5. Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
6. Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. doi:10.1056/NEJMoa1712126

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen