Adjusting to New Therapies in Relapsed/Refractory RCC
At a live, virtual Case-Based Roundtable meeting, Bradley McGregor, MD, discussed with fellow physicians how best to integrate new approaches to treatment for patients with relapsed or refractory clear cell renal cell carcinoma.
Bradley McGregor, MD
Director of Clinical Research
Lank Center for Genitourinary Oncology
Dana-Farber Cancer Institute
Instructor in Medicine
Harvard Medical School
Boston, MA

UPDATED NCCN GUIDELINES SHOW NEW TREATMENT OPTIONS
McGREGOR: I hadn’t realized until recently how much the kidney cancer NCCN guidelines had changed in 2023.1 [I had yet to take in] the fact that all the second-line trials we had weren’t done in the modern era, and they were done [before the] advent of IO.
Now, the NCCN guidelines stratify the subsequent therapies based on whether patients used any prior IO therapy and they’ve also taken away category 1 designations because most of the trials are done in an area when very few patients had IO. For example, [in the] METEOR study [NCT01865747], less than 10% of the patients had prior immunotherapy,2 so while I think that’s still compelling for cabozantinib [Cabometyx], the category designation has been removed. So, if a patient is IO therapy–naïve there’s no preferred regimen; we just have a lot of options.1 They recommend any of the IO combinations that are utilized in the front line, through all the IO and TKI combinations, as well as nivolumab [Opdivo] and ipilimumab [Yervoy].
In addition, targeted therapy alone is an option with cabozantinib and nivolumab. If a patient had prior immunotherapy, the list [of recommended regimens] gets much shorter, including axitinib [Inlyta], cabozantinib, lenvatinib [Lenvima] plus everolimus [Afinitor], and tivozanib.... So it’s a dramatic change in how this is presented, but I think it is a much more useful presentation and also gets to the point of where the data [are] right now.
CHOOSING THIRD-LINE AND SECOND-LINE TREATMENTS
McGREGOR: I think tivozanib stands out. It seems to be highly active against VEGF-R1, but also 2 and 3, maybe more so than some of the other TKIs, and it’s certainly an interesting choice. And tivozanib, while it’s new to the United States, has been around for quite some time in Europe, and there were actually a lot of studies done earlier on with it. [The new use with it is] recently based on the TIVO-3 trial [NCT02627963], which brought it to the United States.3
I find that each TKI is very different. I have patients who don’t tolerate one, but they may tolerate the next perfectly fine. So someone intolerant to cabozantinib may do great with lenvatinib or vice versa, or on axitinib they will tolerate it, but maybe not another, whereas tivozanib can be very well tolerated. Each of these TKIs is different, as they have different MIC50s [half minimum inhibitory concentration] for different receptors, and I think that does come into play in their toxicity pattern, their profiles as well, and the half-life of these drugs are quite different as well.
The dosing strategy may be different, so there are a lot of different factors that come into play when we start thinking about these different TKIs, which is why I think the NCCN has realized that we don’t have great data for a sequential [hierarchy of which is better so] they grouped them together and removed the category 1 recommendations for that very reason, although tivozanib is studied in the third-line and later setting, and that’s where it showed its efficacy vs sorafenib. I think this gets to the point of how we start choosing a treatment overall.
DOSING PRACTICES WITH TIVOZANIB
McGREGOR: The given dose in the study was 1.5 mg, but the recommended dose of tivozanib is now 1.34 mg daily. The standard dose is 1.34 mg given once daily for 21 days, followed by 7 days off, so this [drug] comes in 21-capsule bottles. It’s given until new progression of the disease or unacceptable toxicity, and it does not matter if it’s given with or without food. As with all other TKIs, the initial management is holding and dose reductions. The dose reduction goes down to 0.89 mg, 21 days on treatment, followed by 7 days off treatment as well. I will say…in some of the monotherapies, like the tivozanib and nivolumab trial, they allow it going down to 0.89 mg every other day without a week off.
EVALUATING THE EFFICACY IN TIVO-3
McGREGOR: A clinically relevant proportion of patients were alive and progression free at 3 and 4 years after tivozanib therapy compared with sorafenib. And again, remember, this is a third- or fourth-line therapy and this is consistent across all clinical demographic subgroups [Table4]. After 4 years, we have a difference of 7.6%, so 7.6% of patients in the trial were still on tivozanib at 4 years.
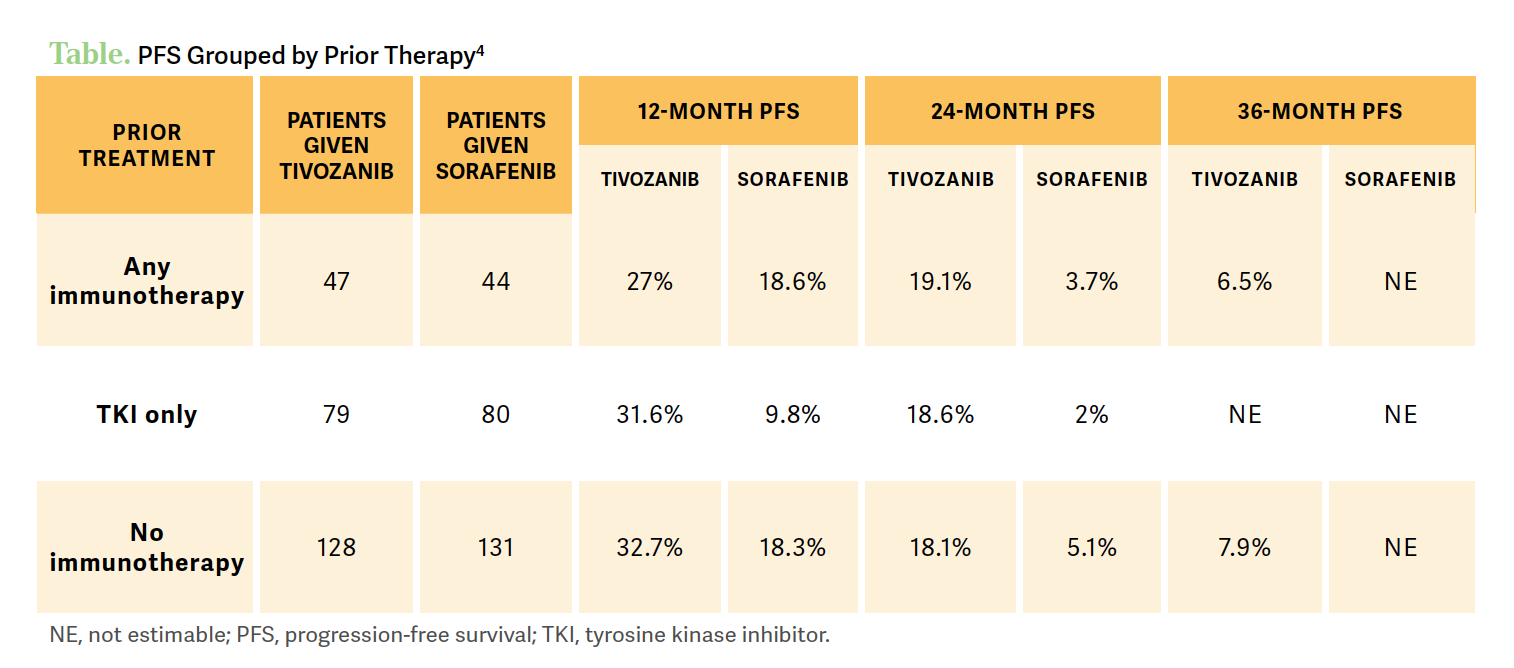
However, a good number of patients in this group had prior immunotherapy, but we see that patients with independent prior immunotherapy had a benefit with tivozanib vs sorafenib at 7.3 months vs 5.1 months of median progression-free survival [PFS], respectively. Looking at that 36-month PFS percentage is right around 10% or higher in these patients. So again, activity is preserved independent of prior immunotherapy, which is important, because that’s what most patients are going to be getting now.
Looking at response, in the third or fourth line, 23% had a partial response on tivozanib vs 11% in the sorafenib arm. The disease control rate was stable over 80% in the tivozanib group. The median duration of response was 20.3 months for patients on tivozanib compared with 9 months in the comparator arm.
Now, for the patients who receive sorafenib who could cross over…the survival has been difficult to directly understand.5 Initially, the overall survival [OS] HR is very close to 1, but with extended follow-up, we see the HR continuing to decline to 0.99 and now it’s down to 0.89 with the most recent data lock. It continues to mature and the thought is with that progression of patients, a 7.6% PFS rate at 48 months may lead to an improvement in OS of the entire population.
References
1. NCCN Clinical Practice Guidelines in Oncology. Kidney cancer, version 4.2023. Accessed June 2, 2023. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
2. Choueiri TK, Escudier B, Powles T, et al; METEOR Investigators. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814-1823. doi:10.1056/NEJMoa1510016
3. Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1):95-104. doi:10.1016/S1470-2045(19)30735-1
4. Atkins MB, Verzoni E, Escudier BJ, et al. Long-term PFS from TIVO-3: Tivozanib (TIVO) vs sorafenib (SOR) in relapsed/refractory (R/R) advanced RCC. Presented at: 2022 American Society of Clinical Oncology Genitourinary Cancers Symposium. February 17-19,2022; San Francisco, CA. Abstract 362.
5. Rini B, Pal S, Escudier B, et al. Maturation of overall survival (OS) in TIVO-3 with long-term follow-up. J Clin Oncol. 2022;40(suppl 16):abstract 4557. doi:10.1200/JCO.2022.40.16_suppl.4557

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen