Leal Examines KRAS-Targeted Agents in Non–Small Cell Lung Cancer
During a Targeted Oncology™ Case-Based Roundtable™ event, Ticiana Leal, MD, discussed the use of sotorasib and adagrasib in patients with KRAS G12C mutations in non–small cell lung cancer.
Ticiana Leal, MD
Associate Professor
Director, Thoracic Medical Oncology Program
Department of Hematology and Medical Oncology
Emory University School of Medicine
Atlanta, GA

CASE SUMMARY
- A 63-year-old woman presents with anorexia, weight loss of 5 lb, fatigue, and dyspnea on exertion. She has medically controlled diabetes and takes a gastric acid-reducing agent as needed.
- A former smoker, she quit 13 years earlier (30 pack-years).
- Laboratory results were within normal limits.
- Chest x-ray showed 2 masses on right lower lobe of lung.
- Chest/abdomen/pelvic CT scan confirmed 2 masses (4.7 cm and 7.4 cm) in right lower lobe and multiple liver lesions.
- PET scan showed liver masses and activity in the right lower lobe.
- Brain MRI was negative.
- Bronchoscopy with transbronchial biopsy of the right lower lobe confirmed lung adenocarcinoma (PD-L1 20%).
- Staging: IVA adenocarcinoma; ECOG performance status (PS) of 1
- Tumor next-generation sequencing (FoundationOne) was positive for KRAS G12C and negative for others.
- PD-L1 expression was 20% per immunohistochemistry (22C3 pharmDx).
- She receives pemetrexed and carboplatin (Alimta) plus pembrolizumab (Keytruda). She achieved a partial response at completion of 4 cycles of chemotherapy. Pemetrexed plus pembrolizumab maintenance was continued.
- Now, 14 months later, while still on pembrolizumab, the patient reports worsening back pain and shortness of breath.
- CT scan shows progression of lung and liver metastases and a new adrenal tumor.
- Repeat MRI of the brain was negative.
What are the National Comprehensive Cancer Network (NCCN) recommendations on molecular testing in non–small cell lung cancer (NSCLC)?
LEAL: The recent version of the NCCN guidelines strongly recommend broader molecular profiling to identify rare driver mutations for which we have effective therapies…or perhaps emerging targets and clinical trials, which are an opportunity for patients as well. They define broad molecular profiling loosely, but basically, it is a molecular test that will give you all biomarkers that are identified, either in sequence in single-gene assays or a combination of a limited number of assays that can also identify beyond the FDA-approved targeted therapy options and perhaps emerging biomarkers as well. To be more inclusive, they do mention tiered approaches based on low prevalence of certain biomarkers as an acceptable strategy.1 Increasingly, we’ve been doing broad molecular testing that encompasses all these targetable alterations in 1 setting.
A patient with metastatic NSCLC who has a tumor with PD-L1 expression of 1% or greater and a targetable driver oncogene should receive frontline targeted therapy for that mutation and not frontline ICI [immune checkpoint inhibitor] because targeted therapy is overall better, with higher response rates and better tolerability. With some of the alterations, we think that the patients are not going to respond to the ICI therapy, with the exception of patients with certain alterations like the KRAS G12C mutation or the HER2 mutation because those patients have targeted therapy approved in the second line and beyond.1
Q: How effectively are health care providers testing for biomarkers in the metastatic NSCLC population?
A real-world study that looked at the use of biomarker testing in a community setting is the MYLUNG trial [NCT05644808]. This is a retrospective chart review study that looked at patients with metastatic NSCLC [who were] initiated on frontline systemic therapy between 2018 and 2020. It highlighted the challenge of doing biomarker testing across the board, including in the community setting. They looked at 5 biomarker tests. We now have more than these 5 [biomarkers] based on the NCCN Guidelines, and we have approved therapies for more than these 5. The study had more than 3000 patients, and of these, almost 3000 had nonsquamous [histology], and 79% of the whole group had 1 biomarker or more tested before receiving frontline therapy.… [Ten percent]…received the biomarker test result after frontline therapy was initiated, and 11% had no biomarker testing. Less than 50%…had all the biomarker tests done.2
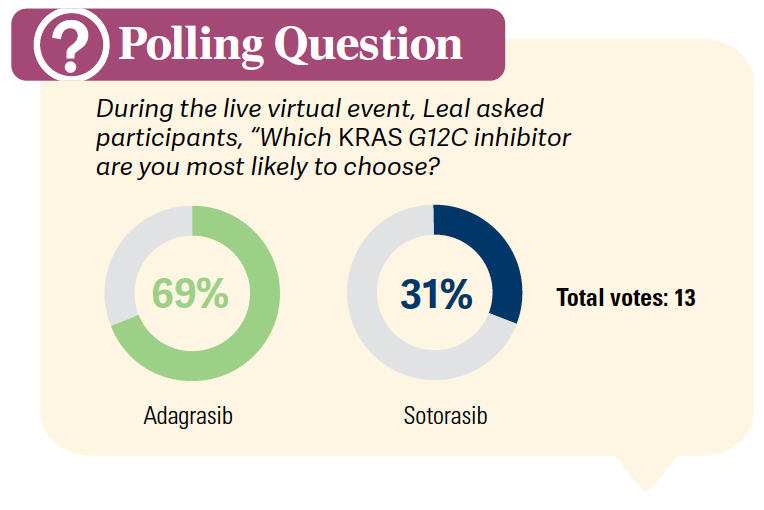
What data support the use of adagrasib (Krazati) in patients with metastatic NSCLC and KRAS G12C mutations?
The study that led to the approval of adagrasib for patients with KRAS G12C mutations is the phase 2 KRYSTAL-1 study [NCT03785249]. The KRAS G12C cohort A [included] patients who had prior therapy with a PD-1/PD-L1 inhibitor in combination or in sequence with chemotherapy. Adagrasib is a KRAS G12C inhibitor that irreversibly and selectively binds to KRAS G12C, locking it in its inactive state. Patients received adagrasib at the 600-mg twice daily dose, and the primary end point was overall response rate [ORR]. Secondary end points were duration of response [DOR], PFS [progression-free survival], overall survival [OS], and safety. This study did allow patients with treated stable CNS [central nervous system] metastases.3
In terms of ECOG PS, 83% of the patients had ECOG PS of 1. These patients were heavily pretreated, as 12% of the patients had 4 previous lines of systemic therapy.3
Patients with previously treated KRAS G12C mutation– positive NSCLC had a response rate of 42.9%, and the disease control rate [DCR] was [79.5%,] with a median DOR of 8.5 months. The median PFS was 6.5 months, and median OS was 12.6 months.3
The study allowed patients with stable and treated brain metastases. Preclinical data showed brain activity of adagrasib in mouse models. A post hoc evaluation of best response in the brain demonstrated activity of adagrasib in the brain, with an intracranial confirmed ORR of 33%, a median duration of intracranial response of 11 months, and a median intracranial PFS of 5.4 months. Of note here, 81.8% of patients received prior radiation before enrolling in this study, and 59% of them were within 3 months of study entry.3
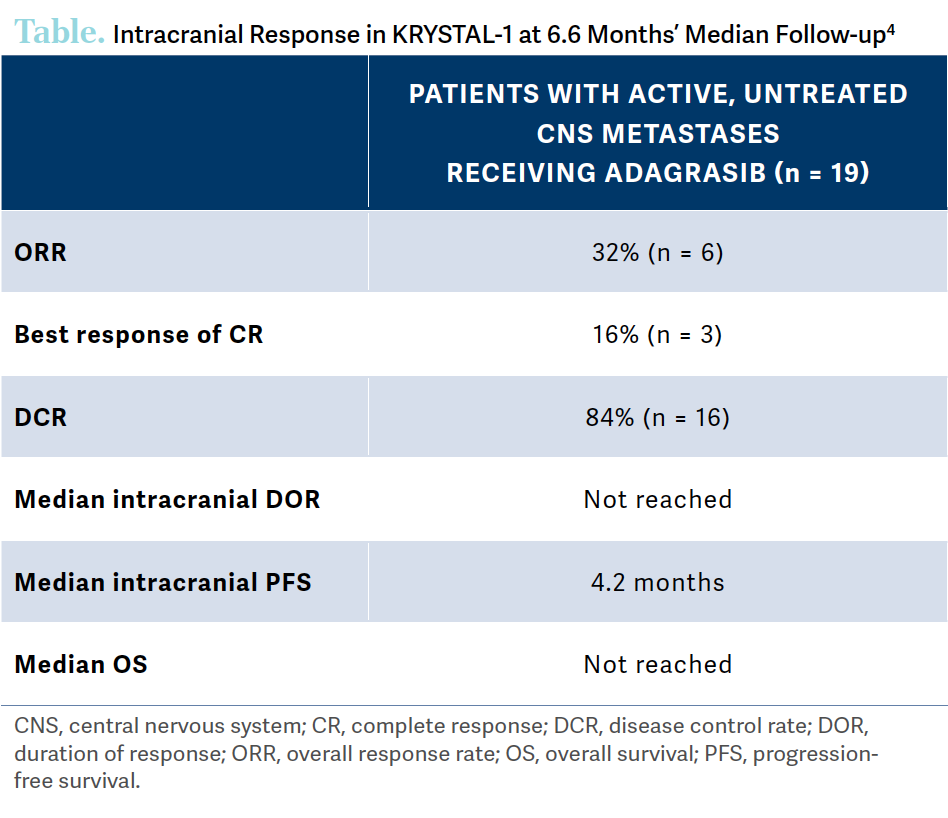
A small cohort of 25 patients with active, untreated CNS metastases were treated with adagrasib to highlight its activity in the brain [Table4]. The ORR was 32%, DCR was 84%, the median intracranial duration of response was not reached, the median intracranial PFS was 4.2 months, and the median OS was not reached.4
In terms of tolerability of adagrasib, adverse reactions were seen in 20% or greater of patients. GI [gastrointestinal] adverse effects [AEs] included diarrhea, nausea, vomiting, constipation, and abdominal pain. Additional AEs were fatigue, edema, musculoskeletal pain, and hepatotoxicity. Grade 3 or 4 AEs were relatively low, with 10% hepatotoxicity, 6% renal impairment, 10% dyspnea, and 17% pneumonia.5
An ongoing phase 3 global study [KRYSTAL-12; NCT04685135] is randomly assigning patients to adagrasib or docetaxel [Taxotere]. Patients have the KRAS G12C mutation and have had prior platinum as well as prior PD-1/PD-L1 inhibitors. They received adagrasib at the FDA-approved dose of 600 mg twice daily or docetaxel at the standard-of-care dose, and it was a 2:1 randomization. The primary end points are PFS and OS, and secondary end points include safety, ORR, duration of response, 1-year survival, etc. This study is ongoing, and we do not have the results as of today.
Q: What data led to the approval of sotorasib (Lumakras) in patients with metastatic NSCLC and KRAS G12C mutations?
Sotorasib, a KRAS G12C inhibitor, was investigated in the phase 2 single-arm CodeBreaK 100 study [NCT03600883], which was done in patients with metastatic NSCLC and a KRAS G12C mutation assessed by tumor biopsy who had progressed on prior therapies. They did allow patients with stable brain metastases. Patients received sotorasib at the now FDA-approved dose of 960 mg once daily until disease progression. The primary end point was ORR. No more than 3 prior lines of therapy were allowed in the study.6
About 69% of patients had ECOG PS 1, 42% of patients had 1 prior line of therapy, 34% had 2 prior lines, and 22% had 3 prior lines, which was the maximum…allowed. About 89% of patients had previous platinum-based chemotherapy, and 81% of patients had both platinum-based and ICI therapies.6
The ORR was 37%, DCR was 80%, median DOR was 11 months, median PFS was 6.8 months, and median OS was 12.5 months.6
A post hoc analysis of sotorasib in patients with stable brain metastases included both patients with target and nontarget stable lesions. The ORR of sotorasib in this population was 25%. This was a small patient population, but the target and nontarget stable brain metastases were used according to the Response Assessment in Neuro-Oncology criteria, and 9.2% of the patients had a baseline and 1 or more on-treatment evaluable brain scans. The brain scans were not mandated throughout the study. The responses in patients with both target and nontarget CNS lesions are quite small. In patients with both target and nontarget CNS lesions, 1 patient had stable disease [SD] and 2 had progression of disease [PD]. In the nontarget CNS lesions group, 2 patients had complete responses [CR], 11 had SD, and 0 had PD. So the post hoc analysis suggests that sotorasib may have brain activity in patients with KRAS G12C mutations.6
All-grade AEs were seen in 15% or greater of patients. Again, GI AEs are the most common, with diarrhea, nausea, vomiting, constipation, and abdominal pain. Hepatotoxicity is also significant. Respiratory AEs included cough and dyspnea. Other AEs were musculoskeletal pain, fatigue, and edema. Grade 3 or 4 AEs that are most common are low in numbers as well, with 5% diarrhea and 12%…hepatotoxicity.7
Q: What additional data support the use of sotorasib?
The CodeBreaK 200 study [NCT04303780] was a global randomized phase 3 study that enrolled patients with advanced NSCLC with a KRAS G12C mutation.
Patients had to have 1 or more prior treatments, including a platinum-based chemotherapy and an ICI. This study allowed patients who had an ECOG PS of 0 to 1, but no active brain metastasis were allowed. Patients were randomized 1:1 to sotorasib or docetaxel. The primary end point was PFS.
This study was presented by Melissa L. Johnson, MD, at the 2022 European Society for Clinical Oncology Congress in Paris. It showed that the study met its PFS primary end point, with an HR of 0.66. The median PFS was 5.6 months vs 4.5 months with docetaxel. However, the OS was not superior with sotorasib, but the study was not powered for OS. The OS for sotorasib was 10.6 months vs 11.3 months with docetaxel. The ORR, DCR, and median DOR were superior for sotorasib compared with docetaxel. The median study follow-up was 17.7 months.8
Overall, the safety of sotorasib is consistent with what we saw in the phase 1 and…phase 2 study and is favorable compared with docetaxel.
Both agents were FDA approved based on phase 2 single-arm studies with ORR as the primary end point.9,10 For adagrasib, the ORR was 43% vs 37% for sotorasib. The median duration of response for adagrasib was 8.5 months vs 11.1 months for sotorasib. The median PFS were similar. The median OS were strikingly…similar, and the [rate of ] treatment-related AEs [TRAEs] was 97% for adagrasib, and 69% for sotorasib. However, the treatment discontinuation rates are quite similar, despite the higher rate of TRAEs with adagrasib.3-7
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Non–small cell lung cancer, version 1. 2023. Accessed April 24, 2023. https:// bit.ly/3It0Dye
2. Robert NJ, Espirito JL, Chen L, et al. Biomarker testing and tissue journey among patients with metastatic non-small cell lung cancer receiving first-line therapy in The US Oncology Network. Lung Cancer. 2022;166:197-204. doi:10.1016/j.lungcan.2022.03.004
3. Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387(2):120-131. doi:10.1056/ NEJMoa2204619
4. Sabari JK, Spira AI, Heist RS, et al. Activity of adagrasib (MRTX849) in patients with KRASG12C-mutated NSCLC and active, untreated CNS metastases in the KRYSTAL-1 trial. J Clin Oncol. 2022;40(suppl 17):LBA9009. doi:10.1200/JCO.2022.40.17_ suppl.LBA9009
5. Krazati. Prescribing information. Mirati Therapeutics Inc; 2022. Accessed April 24, 2023. https://bit.ly/42RgkYa
6. Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371-2381. doi:10.1056/NEJMoa2103695
7. Lumakras. Prescribing information. Amgen Inc.; 2021. Updated May 2021. Accessed April 25, 2023. https://bit.ly/45iF6BW
8. Johnson ML, de Langen AJ, Waterhouse DM, et al. Sotorasib versus docetaxel for previously treated non-small cell lung cancer with KRAS G12C mutation: CodeBreaK 200 phase III study. Ann Oncol. 2022;33(suppl 7):S808-S869. doi:10.1016/ annonc/annonc1089
9. FDA grants accelerated approval to adagrasib for KRAS G12C-mutated NSCLC. News release. FDA. December 12, 2022. Accessed May 19, 2023. https://bit. ly/41Skq0L
10. FDA grants accelerated approval to sotorasib for KRAS G12C mutated NSCLC. News release. FDA. May 28, 2021. Accessed May 19, 2023. https://bit.ly/43dLYyC

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More