PARTICIPANT LIST
Karen C. Daily, DO
Frantz Francisque, MD
Uday Dandamudi, MD
Simon Blanc, MD
Mahender Yellu, MD
Mahdi Taha, DO
Sumithra Vattigunta-Gopal, MD
Seetal Mewar, MD
Georges Azzi, MD
Aaron Denson, MD
Harsh Amin, MD
EVENT REGION Florida
During a Targeted Oncology™ Case-Based Roundtable™ event, Hatem Soliman, MD, discussed how the FDA approval of elacestrant impacts the treatment setting of ER+, HER2- breast cancer
Hatem Soliman, MD (MODERATOR)
Medical Director of the Clinical Trials Office
The Center for Women’s Oncology
Moffitt Cancer Center
Tampa, FL

EVENT REGION Florida
CASE SUMMARY
A 56-year-old postmenopausal woman presented with a palpable right breast mass with no clinically abnormal axillary lymph nodes. A core biopsy led to diagnosis of grade 2, estrogen receptor–positive (ER+)/progesterone receptor–positive (PR+) invasive ductal carcinoma (IDC) with a HER2 immunohistochemistry (IHC) score of 0 and Ki-67 score of 33%. Lumpectomy and sentinel lymph node (SNL) biopsy results showed tumor measuring 3 cm, grade 2 IDC, and 2 SNLs negative for malignant cells. Her 21-gene recurrence score was 27.
The patient received docetaxel (Taxotere) and cyclophosphamide (Cytoxan) for 4 cycles followed by radiation therapy, and she completed 5 years of adjuvant aromatase inhibitor (AI) therapy. Three years later, she reported right-sided abdominal pain and mild nausea. A CT scan of the chest, abdomen, and pelvis showed 3 suspicious liver lesions (right lobe; largest measuring 2 cm). Liver biopsy results showed ER+/PR+ IDC with a HER2 IHC score of 0. Comprehensive molecular testing results from tissue biopsy showed no actionable alterations. AI therapy plus palbociclib (Ibrance) was initiated.
The therapy was well tolerated by the patient, with low-grade neutropenia that did not require interruption. Twenty months later, follow-up imaging shows enlargement of liver nodules and 2 new lung nodules (largest measuring 0.9 cm). Her ECOG performance status is 0.
DISCUSSION QUESTION
SOLIMAN: [Would you] lean toward using abemaciclib [Verzenio] or ribociclib [Kisqali] over palbociclib based on some of the overall survival [OS] data from the MONALEESA-7 [NCT02278120] and MONARCH 3 [NCT02246621] trials? Or are you all still using a lot of palbociclib in the first line?
DAILY: I changed from palbociclib to abemaciclib [because of] the OS data. The positive adjuvant data for abemaciclib, although we don’t have ribociclib [data] yet, also influenced me. So I’ve been starting new patients on abemaciclib.
FRANCISQUE: I [would choose] abemaciclib, but it usually comes down to comorbidities that guide which one I pick.
DANDAMUDI: I’m moving toward abemaciclib, but at the same time, I have good experience with palbociclib; patients tolerate this very well compared [with] the other 2 [medications].
BLANC: I lean more [toward] palbociclib. I tried the other 2 [medications]. In my opinion, it’s a class effect. I don’t think there’s one better than the other. I just found it inconvenient, initially, with ribociclib, to do the ECGs [electrocardiograms]1 in the office. For abemaciclib, I had bad experience with adverse events [AEs].
YELLU: I’ve been using palbociclib for some time; however, [because of] recent data, I’ve been switching to ribociclib. Unless patients have cardiac comorbidities, I’m going with ribociclib. ECGs are a pain.1 I’ve used abemaciclib in the past, [and the patient had a] very bad experience with diarrhea and…could not tolerate it. When they switched back to palbociclib, they did well. But with the recent data, I’ve been using ribociclib more. It looks like the abemaciclib OS data are an interim readout.2
SOLIMAN: Yes. We’re still going through the data, so there could be some differences as far as the OS signal over time. But [they are] interesting, the different perceptions or experiences [regarding] the difficulty of starting the different agents. Dr Taha, do you have any preference?
TAHA: I don’t. I’ve been utilizing [palbociclib] for a long time like everyone else. I’ve been trending toward abemaciclib for a little bit, but the diarrhea has been a bit of a challenge, so I may revert to palbociclib, because I’ve been using it and feel very comfortable with it. Despite the OS data, I feel comfortable with something that I’ve been utilizing for a long time now.
VATTIGUNTA-GOPAL: I use palbociclib because I’m more comfortable with it. That’s the first one that came to the market, and I’m used to the dose reductions and neutropenia. I’ve tried ribociclib and abemaciclib, but I agree with the ECG needing to be done1 and the cardiac [issues]. Abemaciclib does cause diarrhea. You have to be on top of it with the electrolyte imbalances. Even though there are OS data,2 I have not tried to change because of the ease. The toxicity was a little more than what I’ve seen with palbociclib.
MEWAR: I was mostly using palbociclib up front, but now I’ve been using ribociclib, and I use abemaciclib in the second line. Sometimes I feel more confident [about treatment in] the patients in whom I’ve used abemaciclib for the first line, [such as] the ones with visceral disease trying ribociclib afterward with fulvestrant [Faslodex]. I’ve had some short responses. Patients with visceral disease are usually a little worse off to start with, but I’ve had success that way. But I’ve mostly moved away from palbociclib.
CASE UPDATE
Blood-based circulating tumor DNA analysis showed an ESR1 mutation.
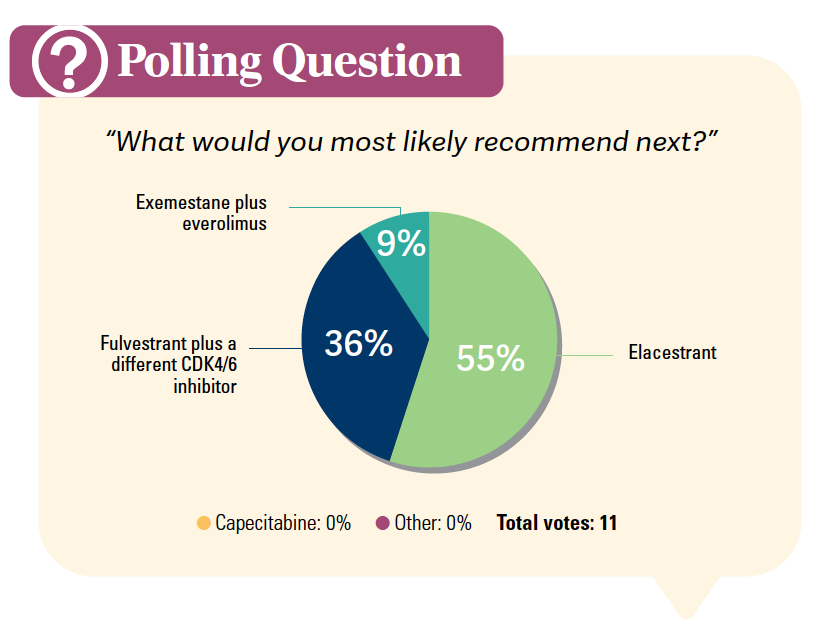
DISCUSSION QUESTIONS
SOLIMAN: What are your thoughts about the results [of elacestrant] for the overall group and the ESR1-mutated [disease] subgroup?
DAILY: My thought is that it’s also effective for the ESR1– wild-type group.3 Maybe not as effective, but it would be nice if the label allowed a broader use.4
BLANC: This is a new standard of care. I’ve seen the data before. It is part of my practice moving forward.
What I like about these breast cancer evolutions is that when CDK4/6 inhibitors came out, you saw tremendous improvements in progression-free survival [PFS] and OS, and now we’re seeing another tremendous improvement. It would be nice to see it in other disease areas where we’re fighting [to gain] a month or 2 of PFS. It’s encouraging.
AZZI: I feel the same, but I would have hoped for a greater magnitude of benefit in terms of PFS. Even for the best responders, the PFS is disappointing.3 There’s nothing better right now, but we need better drugs for the future.
SOLIMAN: Dr Dandamudi, what’s your take on the safety data3,5 as far as your experience with other targeted drugs that are used in the salvage setting?
DANDAMUDI: There is some GI toxicity and fatigue,3,5 and all those things can be easily managed. With all these newer targeted treatments, we are aware of the situation, and we can manage them. But my main [concern] is that there is a rapid drop-off. In the first 2 or 3 months, there is significant drop-off.3 That makes me nervous to start this patient on the oral selective estrogen receptor degrader [SERD].
SOLIMAN: [What about] using the criterion of the patient’s duration of benefit on the prior CDK4/6 inhibitor? For example, a patient who had a long duration of benefit on palbociclib? Do you feel more reassured to start [a SERD] in those patients with ESR1 mutations who have had 16 or 20 months on palbociclib [From the Data5]?

YELLU: That’s an important piece of data: Once a patient progresses, it’s going to be very hard to put them on these endocrine therapies and wait and watch. Sometimes the progression can be rapid. I would be more comfortable if the patient were on CDK4/6 inhibitors for quite some time, maybe 2 or 2.5 years. We know that this biology is better there.5 We’re more comfortable using this drug in this setting. I think the AE profile is not as bad, especially given that this [is an] oral agent, and we know that, going forward, we have more chemotherapy agents. [If] the patient [would] be willing to try this, I would use it.
SOLIMAN: Dr Vattigunta-Gopal, what’s your experience with ET-related fatigue, GI toxicity, or even musculoskeletal pain? We don’t see a lot of GI toxicity with other agents we use. Do you have anything that you use to help with musculoskeletal pain in your patients on AI therapy?
VATTIGUNTA-GOPAL: I try to use nonsteroidal anti-inflammatory drugs [NSAIDs] if possible. I use the NSAIDs sparingly. Sometimes I use meloxicam [Mobic]. I sometimes try to use natural [remedies]; I have even suggested turmeric and other things. Otherwise, if [the musculoskeletal pain] is too much, I tend to refer patients to the rheumatologist to see [whether] there is something else available. With GI toxicity, [there is] not much [available] except using the CDK4/6 inhibitor. I haven’t used an oral SERD. Although we had clinical trials using different SERDs, I have not had experience, so I’m wondering how it will be to use this new oral agent.
SOLIMAN: Dr Denson, [based on] the GI toxicity data,3,5 would you consider using any prophylactic antinausea medicine? If you advise a patient to take the [elacestrant] with food, would you also give them an antinausea medicine up front, or would you have them wait and see?
DENSON: I give [almost] all patients some sublingual ondansetron [Zofran] because it’s easy to do when [individuals] become new patients. That’s part of my routine. It avoids the middle-of-the-night phone call. A lot of patients have it on hand because I give it to everybody.
SOLIMAN: Dr Francisque, as far as the hepatotoxicity3,5 and the monitoring of alanine aminotransferase and aspartate aminotransferase levels, [do they] give you any pause? Is it an issue in your practice, for example, if you were to monitor the patient with liver function tests or if you had to give them prophylactic antinausea medicine? Does any of that concern you?
FRANCISQUE: Not in terms of monitoring the laboratory results considering that there’s dose adjustment [available]. Sometimes it’s an issue with a medication where you cannot adjust the dose, [such as] immunotherapy. So it wouldn’t be a major concern. I’m wondering what will be the [consensus] for a patient who [has a] positive [finding] for ESR1 and PIK3CA [mutations] regarding how we sequence [targeted therapies].
SOLIMAN: [We can] use this case as a vignette [and assume that] this patient [had a] negative [finding] for PIK3CA mutation. There is some ongoing work to look at combining elacestrant with all the combinations, including alpelisib [Piqray], but we don’t have those data yet.
FRANCISQUE: Did the [EMERALD] study exclude patients with PIK3CA mutations?
SOLIMAN: As far as the patients who were treated, a minority had received PIK3CA inhibitors. If you look at the prior treatments, the percentage was less than 1% in some of the arms.3 Does anybody here have any additional perceptions about the ease or difficulty of using elacestrant? Dr Amin, do you feel like elacestrant appears to be practical and easy to use?
AMIN: The GI toxicity is probably manageable, and most patients would take that. They [don’t like] injectable fulvestrant. It’s very painful. So most patients would pursue [treatment with elacestrant]. That said, the drug is of a limited benefit in patients who are endocrine sensitive with an ESR1 mutation,3 so the ones who had prolonged CDK4/6 inhibitor benefit are the ones [ for whom] I will use it. This is probably going to be short-lived because…we may be using this drug in the first line with a CDK4/6 inhibitor.
Discussion Questions
SOLIMAN: Dr Taha, how do you envision the role of AI [therapy] and fulvestrant changing with this new approval?6 How do you think it will shift the landscape?
TAHA: When physicians get familiar with [elacestrant] and feel comfortable with it [regarding] AEs, toxicities, and response rates, it will be utilized, because it will be an advantage [because of the] way it is given to patients if it’s manageable. And patients will request it [rather than] having to come into the office to get injections all the time. It’s favorable for the future.
SOLIMAN: Dr Mewar, what do you see as the pros and cons of elacestrant vs fulvestrant, specifically for patients [such as] the one described in this case?
MEWAR: Elacestrant is not approved to be used with a CDK4/6 inhibitor, so that’s a limitation.6 I usually combine them. [However,] I have one patient who got pneumonitis on a CDK4/6 inhibitor. She was started on letrozole [Femara] and a CDK4/6 inhibitor. She’s on fulvestrant now, and she might be someone for whom I might try [elacestrant].
References
1. Kisqali. Prescribing information. Novartis; 2022. Accessed April 24, 2023. https://www.novartis.com/us-en/sites/novartis_us/files/kisqali.pdf
2. Goetz MP, Toi M, Huober J, et al. MONARCH 3: interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC). Ann Oncol. 2022;33(suppl 7):S808-S869. doi:10.1016/annonc/annonc1089
3. Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246-3256. doi:10.1200/JCO.22.00338
4. Orserdu. Prescribing information. Stemline Therapeutics; 2023. Accessed April 24, 2023. https://bit.ly/3ozccwv
5. Bardia A, Bidard FC, Neven P, et al. GS3-01 EMERALD phase 3 trial of elacestrant versus standard of care endocrine therapy in patients with ER+/HER2- metastatic breast cancer: updated results by duration of prior CDK4/6i in metastatic setting. Cancer Res. 2023;83(suppl 5):GS3-01. doi:10.1158/1538-7445.SABCS22-GS3-01
6. FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer. FDA. January 27, 2023. Accessed April 25, 2023. https://bit.ly/3Lib3m2

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More