Niu Discusses Use of Targeted Therapies in Various Patients With NSCLC
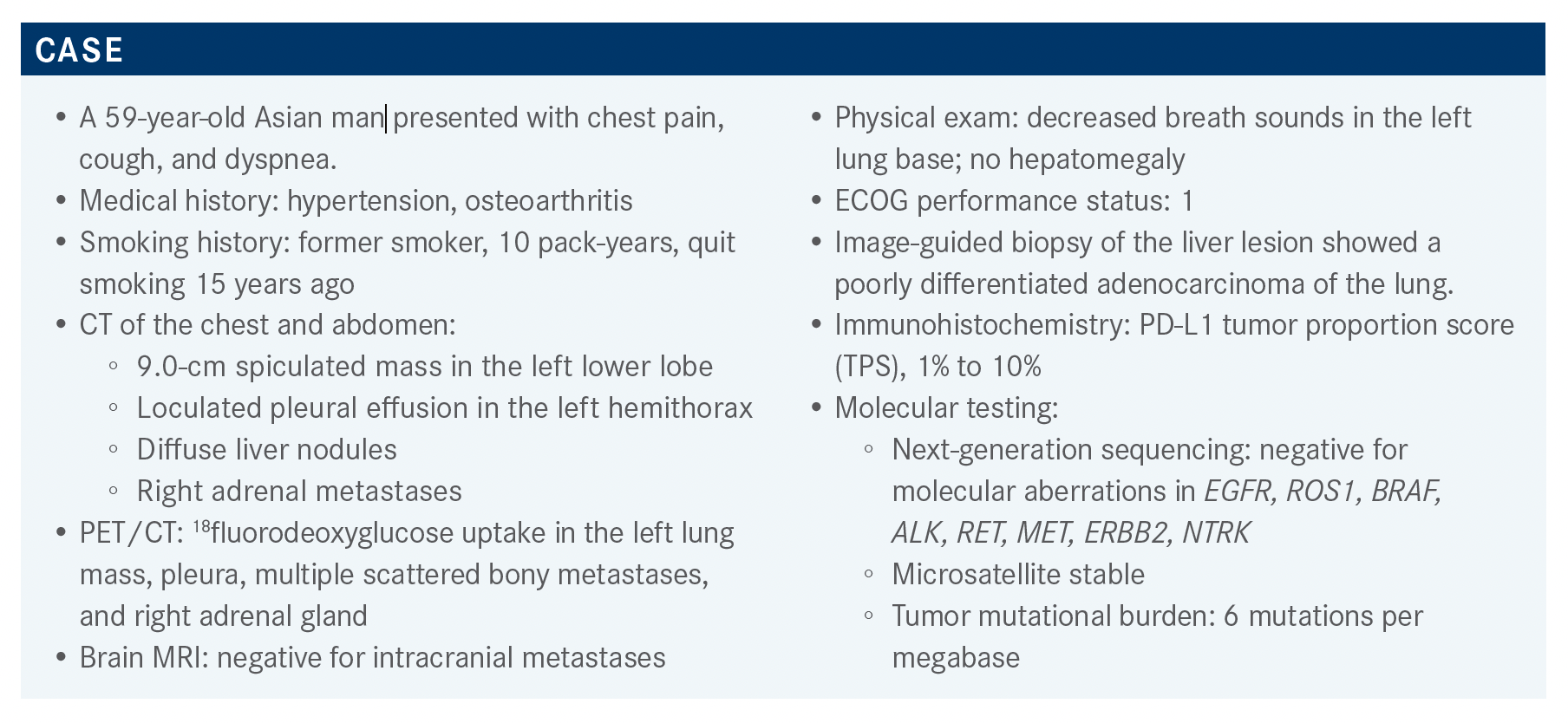
Based on the case of a 59-year-old Asian man with non-small cell lung cancer, Jason Niu, MD discusses the targeted therapy options for the disease.
Jason Niu, MD

Jason Niu, MD, director, Lung Cancer Program and associate director, Head & Neck Cancer Program at Banner MD Anderson Cancer Center, discusses an interesting non-small cell lung cancer patient case.
Targeted Oncology™: What data support the use of pembrolizumab (Keytruda) in metastatic non–small cell lung cancer (NSCLC)?
NIU: The KEYNOTE-189 study [NCT02578680] was based on the KEYNOTE-021 study [NCT02039674] and the PARAMOUNT study [NCT00789373]. All patients received pemetrexed [Alimta] plus either carboplatin or cisplatin.
The experimental arm received pembrolizumab and the control arm received placebo. This regimen was followed by pemetrexed plus either pembrolizumab or [placebo for the experimental and control arms, respectively]. Patients in the control arm who experienced progression were permitted to cross over to the pembrolizumab monotherapy.1
The overall survival [OS] data were published after almost 2 years of follow-up, with very impressive results.
In the experimental arm, the rate of OS was 45.5%, vs 29.9% in the control arm. Median OS was 22.0 months [95% CI, 19.5-25.2 months] vs 10.7 months [95% CI, 8.7-13.6 months], respectively [HR, 0.56; 95% CI, 0.45- 0.70].2 This is quickly becoming a new standard, widely accepted in the United States and in the world.
OS was analyzed in subgroups defined by PD-L1 expression, [as quantified by TPS]. Approximately 25% of patients had a PD-L1 TPS greater than or equal to 50%, and approximately 25% had a TPS less than 1%. The patient [we are considering here has a] TPS that falls within the middle range [1%-49%]. [Within this middle range], the HR for OS was 0.62 [95% CI, 0.42-0.92], and at 24 months, the rate of OS was 44.3% in the experimental arm vs 33.0% in the control arm. These values are very similar to those seen in the overall population, because this [TPS range represented] most of the population in this study.2
Please discuss the poll results.
I think [that when single-agent immune checkpoint inhibitors are used for patients with NSCLC and low PD-L1 expression], usually we are dealing with older patients who have a lot of comorbidities and who may not be candidates for chemotherapy.
Besides the KEYNOTE-189 regimen, are other regimens available? If so, for which patient populations?
One option listed by the National Comprehensive Cancer Network [NCCN] guidelines is the combination of carboplatin, paclitaxel, bevacizumab [Avastin], and atezolizumab [Tecentriq]. This combination is listed as appropriate for patients with nonsquamous disease and PD-L1 expression ranging from 1% to 49%.3 I tend to use the KEYNOTE-189 regimen as a backbone, [but we also] use second-generation [immune] checkpoint inhibitors and [we choose from among] 2 or 3 available options. One checkpoint inhibitor, single-agent pembrolizumab,4 is listed by the NCCN as, [“useful in certain circumstances”].3
What data support the use of single-agent pembrolizumab?
[This regimen is supported by data from] the KEYNOTE- 042 study [NCT02220894]. In this [study, patients had] metastatic or advanced NSCLC with a PD-L1 TPS greater than or equal to 1%. Patients were randomly assigned to receive either pembrolizumab alone or carboplatin combined with either paclitaxel or pemetrexed based on disease histology.5,6
Median OS for patients with high PD-L1 expression, defined as TPS greater than 50% was 20 months [95% CI, 15.4- 24.9] in the experimental arm [HR, 0.69; 95% CI, 0.56-0.85; P = .0003]. For patients with a PD-L1 TPS greater than 20%, median OS was 17.7 months [95% CI, 15.3-22.1] in the experimental arm vs 13.0 months [95% CI, 11.6-15.3 months] in the control arm. The HR was 0.77 [95% CI, 0.64-0.92; P = .0020], not as impressive [as the HR observed for patients with] high PD-L1 expression.5,6
Analysis [of the entire population] revealed a lower median OS at 16.7 months in the experimental arm [95% CI, 13.9-19.7] and a higher HR [HR, 0.81; 95% CI, 0.71-0.93; P = .0018] than those observed for patients with a PD-L1 TPS greater than 50% or greater than 20%.5,6
I want [to note that quite a few] patients dropped out [after beginning] therapy,5,6 which is one of the reasons I don’t like to use [this regimen]. In community [practice], up to 30% of patients may not [survive long enough to begin] second-line therapy and that’s why I’m a big proponent of using combination therapy, though I might be liberal with dose reduction or stop at 2 to 3 cycles.
Exploratory analysis was performed on the population defined by a PD-L1 TPS of 1% to 49%, which is relevant to the patient we are discussing here. The HR was 0.92 [95% CI, 0.77-1.11], so the experimental regimen was not better than chemotherapy.5,6 I think that for this group [of patients], in general, we prefer combination therapy to immunotherapy with single-agent pembrolizumab.
Are there data that support the addition of atezolizumab to any NSCLC regimen?
In the IMpower150 study [NCT02366143], patients in arm A received a chemotherapy doublet, carboplatin plus paclitaxel, plus atezolizumab; patients in arm B received that triplet plus bevacizumab; and patients in arm C received the chemotherapy doublet plus bevacizumab. The study included 120 patients with EGFR mutations, about 40 such patients in each arm. Maintenance therapy was bevacizumab alone, atezolizumab alone, or both bevacizumab plus atezolizumab.7,8 The final analysis compared arm B with arm C. Adding atezolizumab to the triplet therapy provided OS benefit [HR, 0.78; 95% CI, 0.64-0.96; P = .02].
Patients [who exhibited EGFR mutation] and who failed first-line targeted therapy showed survival benefit in the subset analysis.7,8 I tend to use [this regimen] in patients with EGFR mutations. [The authors] mentioned that might work better if a patient has liver metastases,7,8 though I have never used it for that purpose.
Are there clinical trial data to support the use of the nivolumab plus ipilimumab combination?
The CheckMate 227 study [NCT02477826] was a complex study, but I will focus on the analysis comparing the nivolumab [Opdivo] plus ipilimumab [Yervoy] combination with chemotherapy.9,10
At 4 years of follow-up, in the population with a PD-L1 TPS greater than or equal to 1%, the rate of OS was 29% in the experimental arm vs 18% in the control arm [median OS, 17.1 vs 14.9 months, respectively; HR, 0.76; 95% CI, 0.65-0.90]. Among patients with a PD-L1 TPS greater than or equal to 50%, the rate of OS was 37% vs 20%, respectively [median OS, 21.2 vs 14.0 months, respectively; HR, 0.66; 95% CI, 0.52-0.84].11 Very impressive results.
My interpretation is that when you give nivolumab up front, the results are negative, [as was demonstrated in] CheckMate 026 [NCT02041533].12 I think you have to use [nivolumab in] combination, as in CheckMate 227, in which ipilimumab was added to nivolumab.11
In contrast, consider the KEYNOTE-598 study [NCT03302234], in which pembrolizumab alone [was compared with] pembrolizumab plus ipilimumab [in patients with a PD-L1 TPS greater than 50%]. The results from that study were completely negative.13
I don’t think there [are many data to support adding] ipilimumab to pembrolizumab, although both are PD-L1 inhibitors.4,14
Among the CheckMate 227 patients with a PD-L1 TPS less than 1%, at 4 years, the rate of OS was 24% for the experimental arm and 10% for the control arm, which are very respectable data [HR, 0.64; 95% CI, 0.51-0.81].11
The primary end point of this study was OS in PD-L1–positive patients,9,10 but I think these results in patients who are negative for PD-L1 expression are more impressive.
The FDA did not approve this use because it was [studied as] an exploratory end point, but you could consider using [this regimen] off label. Many people are concerned about treatment-related adverse effects [AEs] when you use a combination therapy.
Complications related to immunotherapy are increased; in the past, this was particularly true with ipilimumab. In this study, treatment-related serious AEs of grade 3 and grade 4 were observed in 10.7% of the patients in the control arm vs in 18.4% of those in the experimental arm, which is quite high.15
In my experience, ipilimumab tends to cause gastrointestinal [GI] toxicity and increases the risk of pneumonitis and hypophysitis because ipilimumab binds to the cytotoxic T-lymphocyte antigen 4 [CTLA-4] receptor in the pituitary gland.16
How has the nivolumab plus ipilimumab combination performed when used with chemotherapy for these patients?
CheckMate 9LA [NCT03215706] compared chemotherapy, [chosen according to histology], with chemotherapy plus nivolumab and ipilimumab. In this study, the dose of ipilimumab was [quite low] at 1 mg/kg. The results, first presented at the 2020 American Society of Clinical Oncology Annual Meeting, were positive with a median OS of 15.8 months in the experimental arm vs 11.0 months in the control arm [HR, 0.72; 95% CI, 0.61-0.86].17,18
This study made some inroads, though I think a lot of us have not [yet] accepted this regimen. Toxicities are always a concern when you use [ipilimumab and nivolumab]. Even though the ipilimumab dose in this study was low, there were still substantial grade 3 or 4 treatment-related AEs in the experimental arm: Skin-related AEs affected 4% of patients; endocrine-related AEs, including hypophysitis and adrenal insufficiency, [were] at 3%; and GI-related AEs at 6%.18,19
For patients with high PD-L1 expression, how do the data for single-agent pembrolizumab (KEYNOTE- 598) compare with the data for ipilimumab plus nivolumab (CheckMate 227)?
The [median] OS with single-agent pembrolizumab was longer, about 30 months 13 vs 21.2 months.11 In the KEYNOTE-598 study, the curves for the pembrolizumab arm and the pembrolizumab plus ipilimumab arm were superimposable.13
[I am confident] that, for patients with high PD-L1 expression, if you use pembrolizumab, there is probably no reason to use ipilimumab plus nivolumab. I do think [the combination of ipilimumab and nivolumab] is better than nivolumab alone, but single-agent nivolumab is not approved for this population. [In contrast], data from the IMpower110 study [NCT02409342] support the use of atezolizumab as a single agent.20
[From among the single-agent options], I think most of us use pembrolizumab. My personal preference is not to use single agents when a patient is symptomatic as I’m [not confident] that pembrolizumab will be [sufficient]. I tend to start with a combination, and I tend not to use a single agent unless the patient is at a reasonable age and not symptomatic, or tumor growth is very slow.
In that case, I feel more comfortable [using a single agent]. However, remember that in the KEYNOTE-189 study, after 4 cycles of [treatment that included chemotherapy], the 2 agents [pembrolizumab plus pemetrexed] were used as maintenance therapy.1
REFERENCES:
1. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al; KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi:10.1056/NEJMoa1801005
2. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. J Clin Oncol. 2020;38(14):1505-1517. doi:10.1200/JCO.19.03136
3. NCCN. Clinical Practice Guidelines in Oncology. Non–small cell lung cancer, version 7.2021. Accessed November 15, 2021. https://bit.ly/3FBzNA2
4. Keytruda. Prescribing information. Merck Sharp & Dohme Corp; 2021. Accessed November 18, 2021. https://bit.ly/3HKlMSw
5. Lopes G, Wu YL, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(suppl 18):LBA4. doi:10.1200/JCO.2018.36.18_suppl.LBA4
6. Mok TSK, Wu YL, Kudaba I, et al; KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. doi:10.1016/S0140-6736(18)32409-7
7. Socinski MA, Jotte RM, Cappuzzo F, et al. Overall survival (OS) analysis of IMpower150, a randomized Ph 3 study of atezolizumab (atezo) + chemotherapy (chemo) ± bevacizumab (bev) vs chemo + bev in 1L nonsquamous (NSQ) NSCLC. J Clin Oncol. 2018;36(suppl 15):9002. doi:10.1200/JCO.2018.36.15_suppl.9002
8. Socinski MA, Jotte RM, Cappuzzo F, et al; IMpower150 Study Group. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. doi:10.1056/NEJMoa1716948
9. Hellman MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab (nivo) + ipilimumab (ipi) vs platinum-doublet chemotherapy (PT-DC) as first-line (1L) treatment (tx) for advanced non-small cell lung cancer (NSCLC): initial results from CheckMate 227. Cancer Res. 2018;78(suppl 13):CT077. doi:10.1158/1538-7445.AM2018-CT077
10. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden.N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
11. Paz-Ares LG, Ciuleanu TE, Lee JS, et al. Nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for advanced non-small cell lung cancer (NSCLC): 4-year update from CheckMate 227. J Clin Oncol. 2021;39(suppl 15):9016. doi:10.1200/JCO.2021.39.15_suppl.9016
12. Carbone DP, Reck M, Paz-Ares L, et al; CheckMate 026 Investigators. First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426. doi:10.1056/NEJMoa1613493
13. Boyer M, Şendur MAN, Rodríguez-Abreu D, et al; KEYNOTE-598 Investigators. Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥50%: randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol. 2021;39(21):2327-2338. doi:10.1200/JCO.20.03579
14. Yervoy. Prescribing information. Bristol Meyers Squibb Company; 2021. Accessed November 18, 2021. https://bit.ly/3HJMr1N
15. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
16. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98-106. doi:10.1097/COC.0000000000000239
17. Reck M, Ciuleanu TE, Cobo M, et al. First-line nivolumab (NIVO) plus ipilimumab (IPI) plus two cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients with advanced non-small cell lung cancer (NSCLC): two-year update from CheckMate 9LA. J Clin Oncol. 2021;39(suppl 15):9000.
18. Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211. Published correction appears in Lancet Oncol. 2021;22(3):e92.
19. Reck M, Ciuleanu TE, Cobo Dols M, et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol. 2020;38(suppl 15):9501. doi:10.1200/JCO.2020.38.15_suppl.9501
20. FDA approves atezolizumab for first-line treatment of metastatic NSCLC with high PD-L1 expression. FDA. May 18, 2020. Accessed November 17, 2021. https://bit.ly/3oV7Xbr
