Cooper Analyzes the Different Data in Transplant-Eligible Multiple Myeloma
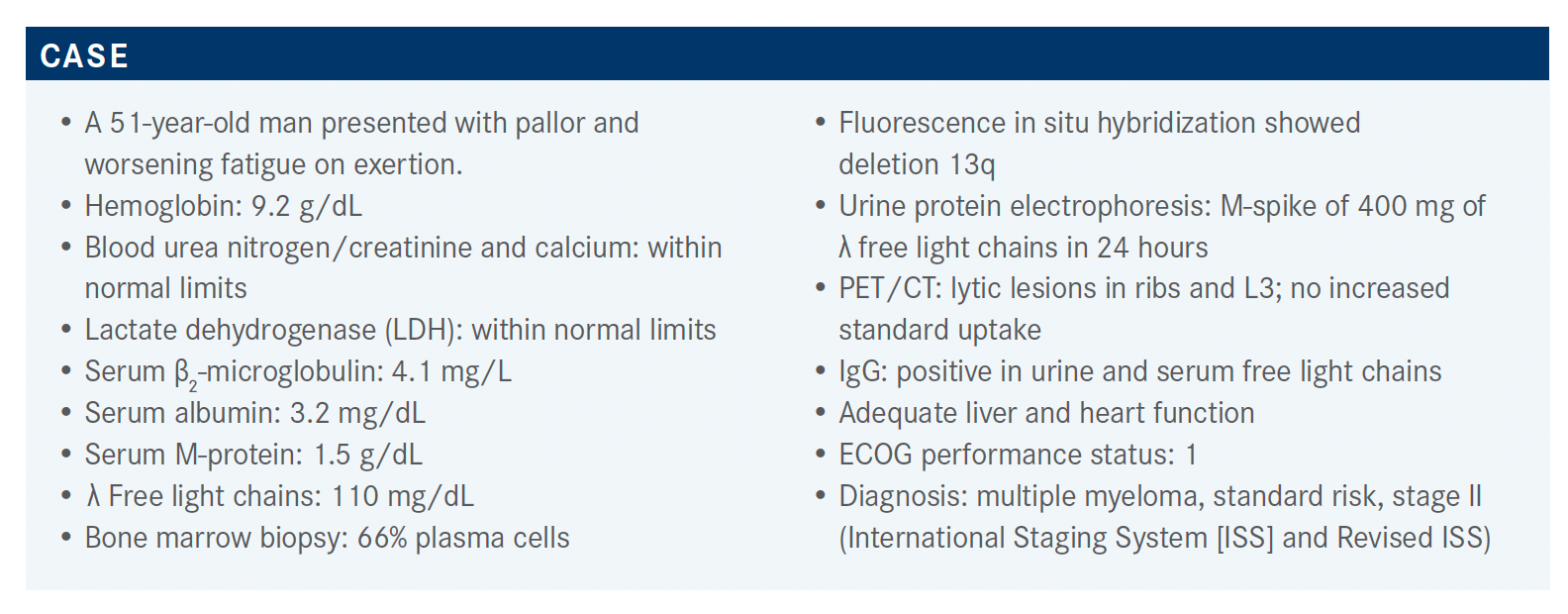
A 51-year-old man presented with pallor and worsening fatigue on exertion.

Dennis Cooper, MD of Rutgers Cancer Institute of New Jersey, discussed the case of a 51-year-old man with transplant-ineligible multiple myeloma during a Targeted Oncology Case-Based Roundtable event.
Targeted OncologyTM: What trial data influence the choice of transplant in patients with newly diagnosed multiple myeloma who are transplant eligible?
COOPER: The IFM 2009 trial [NCT01191060] compared RVd [lenalidomide (Revlimid), bortezomib (Velcade), dexamethasone] plus or minus autologous stem cell transplant [ASCT]. This is one of the very important trials because it most mimics the therapy that we’ve been giving for the past few years. Like many trials done in Europe, there’s a consolidation [phase].
For the point of discussion, we can pretend the patients got RVd for 3 cycles followed by stem cell collection and ASCT, and then they got RVd for cycles 4 and 5 consolidation before [lenalidomide] maintenance for the ASCT arm and then in the other arm there was RVd for 3 cycles, stem cell collection, and then RVd for another 5 cycles [and then lenalidomide maintenance].1,2
Basically, this was a study that looked at ASCT after 3 cycles vs at the time of disease progression [PD]. There are a couple of pieces of very good news. One is that if you look at the 8-year overall survival [OS] rate, it looks like more than 60% of patients—even if they’re transplanted early or late—will be alive at 8 years.
I think many patients look on the internet, they see 2 or 3 years [for survival], and so it’s good to be able to tell them that the data now show that more patients than not are going to be alive at 8 years. We’re routinely seeing 10-year survival.1,2
The second piece of good news is that patients weren’t compromised in terms of OS by delaying ASCT until PD. One impressive thing about this trial, which I don’t think I can match in my practice, is that out of the patients who were not transplanted up front, almost 80% eventually received an ASCT at the time of PD. One of the problems is that if a patient isn’t given transplant at the time of initial presentation, they may not get transplant at all, but this trial clearly indicates otherwise.2
I think one of the potential reasons for considering up-front ASCT is that the median progression-free survival [mPFS] is 47.3 months, which is about a year longer, vs 35 months for the non-ASCT group. The other impressive thing is that of the patients who received up-front ASCT, about 35% were still without relapse at 8 years.2 That’s an impressively long disease-free survival. The standard-risk group and, not quite as much, the high-risk group seem to benefit by getting ASCT. The 2 outstanding things for early ASCT are a longer PFS and the fact that about one-third of the patients had not relapsed 8 years out from their initial treatment.
Can you discuss the role of minimal residual disease (MRD) in this setting?
[First], I want to talk about a few definitions, because in multiple myeloma it can be complicated. Complete remission [CR] means that the patient has a negative serum and urine immunofixation, and less than 5% plasma cells in bone marrow.
A stringent CR [sCR] has, in addition to negative immunofixation, a normal free light chain ratio and the bone marrow shows no clonal plasma cells. So if you have 1% or 2% clonal plasma cells, you can have CR but not sCR unless you have a negative marrow flow and a normal free light chain ratio.
MRD on the other hand is measured with either next-generation flow cytometry or, more commonly in this country, next-generation sequencing [NGS], which look for clonal plasma cells, at more than 1 × 10-5 or 1 × 10-6.
So they measure different things, but currently the gold standard is MRD primarily with NGS, using tests like clonoSEQ assay. Patients who achieved MRD negativity—and it didn’t matter how they got there—did better than patients who did not achieve MRD negativity, even with ASCT. MRD negativity seems to trump the type of treatment one got.3
However, MRD negativity with ASCT had a better PFS than the MRD negativity achieved without ASCT.3 This indicates that there’s a concept of what we call sustained MRD, meaning the patient isn’t just MRD negative at one point in time, say at the end of ASCT, but they maintain their MRD negativity through 6 to 12 months.
My take-home message is that it’s not enough to say that the patient achieved MRD negativity because there’s a deeper level of MRD negativity after ASCT compared with conventional therapy.
For a patient with newly diagnosed multiple myeloma who is transplant eligible and becomes MRD negative at the end of induction, will they still need ASCT, or can it be skipped?
That has been the key question. The patients who achieved MRD negativity with RVd and maintenance did not have as long a PFS as the patients who achieved MRD negativity with ASCT. I think there was this idea that because they achieved MRD negativity, maybe they didn’t need ASCT, but so far that has not completely held up because it seems that if you don’t have ASCT, you do not have as high a likelihood of sustained MRD negativity.
The FORTE trial [NCT02203643] had 1 arm of KCd induction [carfilzomib (Kyprolis), cyclophosphamide, dexamethasone] plus ASCT and KCd consolidation vs 2 arms of KRd [carfilzomib, lenalidomide, dexamethasone]. KCd is basically a modern version of CyBorD [cyclophosphamide, bortezomib, dexamethasone]. So, instead of giving bortezomib, you’re giving carfilzomib. That was one of the arms. The KRd arms are probably the most important, in terms of the long-term aspects of the study. In both arms, they received 4 cycles of KRd, stem cell mobilization, and then one group went on to ASCT [plus KRd consolidation] and the other arm went on to get 8 more cycles of KRd. There was a second randomization [for maintenance with] lenalidomide [R] vs carfilzomib plus lenalidomide [KR].4,5
When these data were first presented, one of the first things the investigators said was that the MRD after the 12 cycles of KRd was as good as the MRD after KRd followed by ASCT and both were superior to getting KCd followed by ASCT. One of the things [individuals] always talk about is the KRd plus or minus ASCT. One of the things that’s striking about this study is that even though the KCd arm got ASCT, they had an inferior outcome compared with the KRd with or without ASCT.4 When we first started doing ASCT, it didn’t seem to matter what the induction [regimen] was. ASCT was the centerpiece of the whole thing. Based on this study, you could say that the KCd indicates a [worse] outcome. The KRd plus ASCT arm still had a better outcome than the 12 cycles of KRd and even though the MRD rates were very similar, the PFS was not as good without ASCT. It indicates that the deepness or the sustainability of the MRD was probably less in the group that did not get ASCT.4,5
After ASCT [and consolidation] or after 12 cycles of KRd, there was a second randomization to R vs KR maintenance. Patients who got the KR had a better PFS. It didn’t matter whether the patients had high-risk cytogenetics or elevated LDH—they still did better. So, the 3-year PFS favored the use of KR vs R alone. However, one of the problems with using that combination is that there were also more adverse events [AEs]. I’m not sure that giving carfilzomib in that type of schedule at maintenance would be all that satisfying for a lot of patients. But, at least in terms of proof of principle, it indicates that patients still benefit from getting more active maintenance therapy compared with R alone.5
What data show efficacy for quadruplet regimens in this space?
[The phase 3 CASSIOPEIA study (NCT02541383) is important for] historical reasons, because I don’t think I’ve given thalidomide [Thalomid] in the last 10 years. It is a European study where they compared VTd [bortezomib, thalidomide, dexamethasone] vs VTd plus daratumumab [Darzalex; D-VTd]. They gave them for 4 cycles [in both arms], then they did stem cell mobilization, conditioning, and ASCT. Like many European trials, they gave further consolidation [for 2 cycles]. There was then a randomization between getting [maintenance] daratumumab every 8 weeks for a maximum of 2 years vs observation alone [until PD]. This is a study that started many years ago before maintenance therapy became the standard of care, but at this time it was not, so it was a very reasonable thing to compare.6
The sCR—which again means negative urine or serum immunofixation, completely negative bone marrow, no clonal plasma cells seen, and normal free light chain ratio— was statistically significantly higher in the group that got D-VTd [at 29% vs 20% for VTd alone (P ≤ .001)]. Just to show that MRD is much different than sCR, the group that got D-VTd had a 64% MRD-negative rate compared with the VTd, which was 44% [P < .0001]. In MRD and sCR, you’re measuring different things and the MRD is going to depend upon what type of assay you’re using. But clearly, this showed a superior result with the addition of daratumumab.6
When you proceed from induction to ASCT to consolidation and then maintenance, the sCR rate continues to deepen over time. Similarly, the MRD-negative rates after ASCT favors the use of daratumumab. One thing that I thought was very interesting, which I think is going to make us look at the GRIFFIN trial [NCT02874742], is when they looked at the maintenance with daratumumab vs nothing. Daratumumab always seemed to benefit the patients who had not gotten it as part of the initial therapy. So if you got D-VTd as your induction, then ASCT, then consolidation with daratumumab, you did not seem to benefit by getting daratumumab as maintenance. The group that benefited, as you might expect, was the [patients] who had not used daratumumab before. The D-VTd group had a better PFS than the VTd alone [HR, 0.47; 95% CI, 0.33-0.67; P < .0001].6 This is one of the reasons why daratumumab got approved as part of the frontline therapy, but unfortunately because of the thalidomide it was not a very popular approach in the United States.
An update from the 2021 American Society of Clinical Oncology Annual Meeting looked at the second randomization and the daratumumab mPFS was better than the group that did not get any further maintenance therapy [HR, 0.53; 95% CI, 0.42-0.68; P < .0001]. However, this PFS [benefit] appeared only in patients who did not receive daratumumab as part of the induction phase.7 That’s an important point.
How did GRIFFIN assess the use of daratumumab in patients with multiple myeloma?
GRIFFIN was a very important trial that was published and has rapidly become the standard of care in many places. They gave [4 cycles of] RVd plus daratumumab [D-RVd] vs RVd alone and then went to ASCT [followed by consolidation]. There was a second randomization [for maintenance therapy] with daratumumab plus lenalidomide [DR] vs lenalidomide [R] alone. The maintenance part of this therapy has not been analyzed yet.
The median age was about 60; 15% were high risk; 14% were International Staging System [ISS] III. There was a lower ASCT rate in the group that got RVd, due to early discontinuation.8 Presumably, that early discontinuation was due to an increased number of patients with PD, which was very uncommon in the group that got daratumumab as part of the initial therapy.
Similar to the CASSIOPEIA study, over time the percentage of patients who get an sCR improves through induction, end of ASCT, end of consolidation, and then after 12 months of maintenance therapy. This is better than what we see with the patients who just get RVd. So at the end of 12 months of maintenance, you have almost 64% who have achieved sCR with D-RVd compared with 47% in the group that got RVd alone.9 The majority of [patients] appear to benefit by getting D-RVd. However, it is interesting that the patients who had high-risk
disease, which in most of these studies [is translocations (4;14) and (14;16), or deletion 17p, and in some studies translocation (14;20)], didn’t look like they benefited in sCR that much by the addition of the daratumumab. Similarly, for MRD negativity rates in high-risk cytogenetics, the patients had a very slight improvement by the addition of daratumumab. The majority of patients with multiple myeloma are doing very well with modern therapy, but there’s still that subgroup that almost seems to have not benefited.8 I think this study, again, would indicate it. It’s a fairly good number of patients that indicates that we’re not quite benefiting the high-risk patients by the addition of daratumumab, like we are in the more standard-risk group.
After a very short follow-up, the PFS and OS were good in both the RVd and D-RVd groups, but it may start favoring the addition of daratumumab [with more time].8
What are the toxicities when adding daratumumab to the RVd regimen?
Many of the trials are showing the same thing. If you focus on grade 3 to 4 AEs, neutropenia, thrombocytopenia, and leukopenia were basically doubled in the group that got daratumumab. It’s not entirely clear why. We don’t think of daratumumab as being a myelosuppressive drug and I think it’s important not to dose-reduce the daratumumab, but to be aware that when you’re adding daratumumab, there is twice the risk of neutropenia and thrombocytopenia compared with the regimen without.8,9 I didn’t see a major signal that there was a dramatic change in any of the nonhematologic AEs with the addition of daratumumab.
Overall, how do regimens you discussed compare?
In the IFM 2009 trial, the rate of patients who got postconsolidation VGPR [very good partial response] was 78% for [RVd plus ASCT] vs 69% [for RVd alone]. The FORTE study is very interesting because it’s almost as good as what we see with the GRIFFIN trial. It had 89% for KRd plus ASCT vs 87% for KRd alone. Not a major difference there. The CASSIOPEIA study was 83.4% [for D-VTd vs] 78% [for VTd alone]. For the GRIFFIN trial it was 90.9% for D-RVd vs 73.2% for RVd alone.2,5-8
What are we going to see by combining daratumumab with KRd? Because that would look like the logical next step if you’re trying to go for MRD. There have been single-institution trials published with that, which look very favorable, but the data are not mature yet. This is a moving target, but what you can see is that with the addition of daratumumab, there’s a significant increase in the response and PFS.
REFERENCES
1. Attal M, Lauwers-Cances V, Hulin C, et al; IFM 2009 Study. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311-1320. doi:10.1056/NEJMoa1611750
2. Perrot A, Lauwers-Cances V, Cazaubiel T, et al. Early vs late autologous stem cell transplant in newly diagnosed multiple myeloma: long-term follow-up analysis of the IFM 2009 Trial. Blood. 2020;136(suppl 1):39. doi:10.1182/blood-2020-134538
3. Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456-2464. doi:10.1182/blood-2018-06-858613
4. Gay F, Cerrato C, Petrucci MT, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: results from the FORTE trial. J Clin Oncol. 2019;37(suppl 15):8002. doi:10.1200/JCO.2019.37.15_suppl.8002
5. Gay F, Musto P, Scalabrini DR, et al. Survival analysis of newly diagnosed transplant-eligible multiple myeloma patients in the randomized Forte trial. Blood. 2020;136(suppl 1):35-37. doi:10.1182/blood-2020-136907
6. Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29-38. Published correction appears in Lancet. 2019:SO140-6736(19)31403-5
7. Moreau P, Hulin C, Perrot A, et al: Daratumumab maintenance or observation after treatment with bortezomib, thalidomide, and dexamethasone ± daratumumab and autologous stem cell transplant in patients with newly diagnosed multiple myeloma: CASSIOPEIA Part 2. J Clin Oncol. 2021;39(suppl 15):8004. doi:10.1200/JCO.2021.39.15_suppl.8004
8. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936-945. doi:10.1182/blood.2020005288
9. Kaufman JL, Laubach JP, Sborov D, et al. Daratumumab (DARA) plus lenalidomide, bortezomib, and dexamethasone (RVd) in patients with transplant-eligible newly diagnosed multiple myeloma (NDMM): updated analysis of Griffin after 12 months of maintenance therapy. Blood. 2020;136(suppl 1):45-46. doi:10.1182/blood-2020-137109
