Roundtable Discussion: Dietrich and Santos Look at Targeted Treatments for Patients With NSCLC
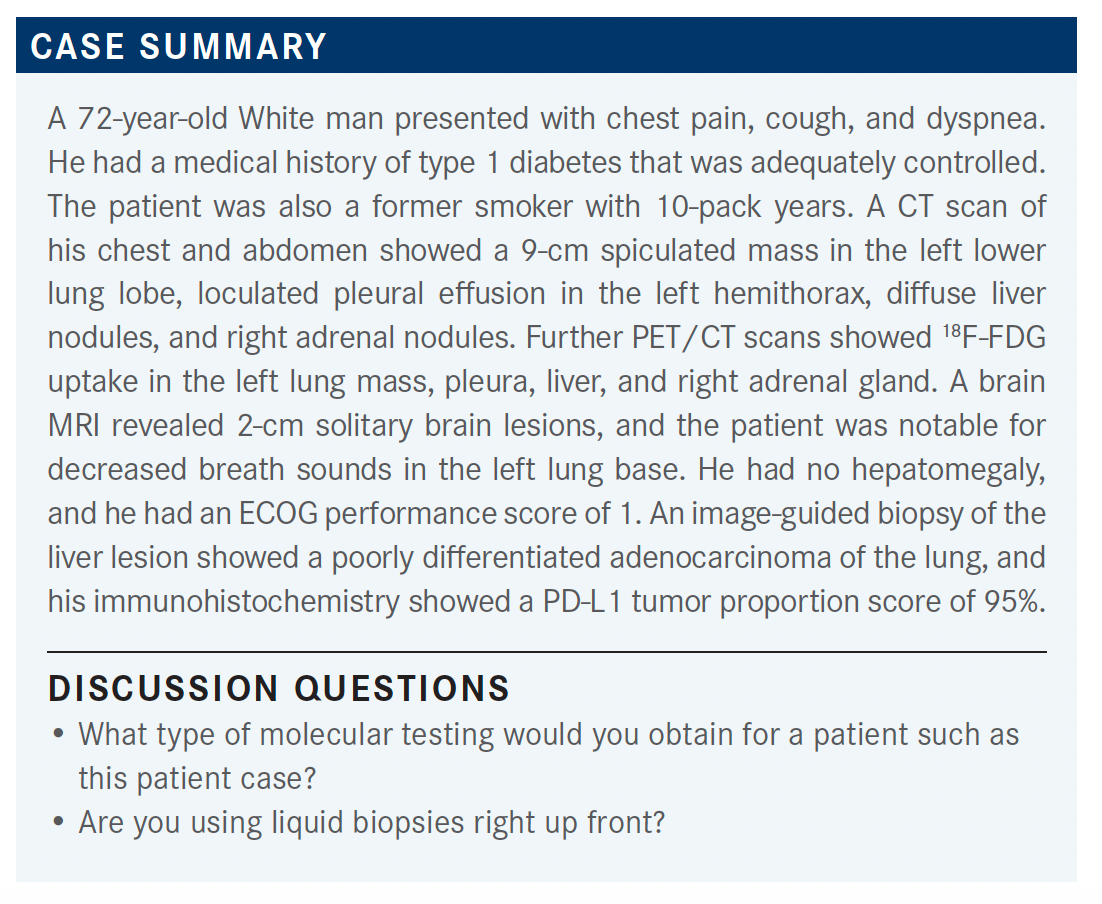
A 72-year-old White man presented with chest pain, cough, and dyspnea. He had a medical history of type 1 diabetes that was adequately controlled, and was a former smoker.
Martin F. Dietrich, MD


A Targeted Oncology Case-Based Roundtable event was co moderator by Martin F. Dietrich, MD, PhD, hematologist/oncologist, Florida Cancer Specialists & Research Institute, and assistant Professor, Internal Medicine, University of Central Florida and Edgardo S. Santos, MD, founding partner, Florida Precision Oncology R&C, FPO, a Division of 21st Century Oncology Thoracic Oncology, clinical affiliate associate professor, Charles E. Schmidt College of Medicine, Florida Atlantic University secretary, FLASCO.
The 2 physicians dusccused the case of 71-year-old patients with non–small cell lung cancer.
RAEZ: At our cancer center, because we do a lot of liquid biopsy research, we do concomitant liquid and tissue biopsy at the same time. The benefit there is that you get the liquid result in 8 or 9 days, and you get the tissue result in 3 weeks. In more than 200 patients, we make the decision based on the liquid biopsy [70% of the time].
DIETRICH: Have you used the reflex option from Guardant Health, where you do the liquid biopsy first, then they run the tissue reflexively in-house?
RAEZ: No.
HURTADO: I usually try to use liquid biopsy as much as I can. In the type of practice that we have, sometimes they get a small sample [of the tissue]. They get a needle biopsy, and depending on what labs you use, they might not have a panel. They might do whatever marker is easier for them and exhaust the tissue. I find myself using liquid biopsy often, sometimes twice 8 weeks apart, to see what I catch.
SHARMA: When the patient comes in, I usually send for Guardant or Foundation at the same time. If for some reason the tissue is inadequate, I wait until I get the liquid biopsy. If there is a driver mutation already there, I stop. If there is not or you need more tissue, then go ahead and get more tissue. That way, liquid biopsy has made life a little easier to find driver mutations. But we do both, and that’s of a standard practice with all my colleagues.
DIETRICH: Is there any particular molecular test you prefer on tissue?
RAEZ: We’ve had a protocol for RET for the past couple years. I prefer RNA-based tissue, and Caris is running an RNA-based platform so we can catch these fusions more accurately. We also had protocols for NTRK before, so that’s why my concern was to catch fusions. That’s why I preferred a platform that runs DNA and RNA.
DIETRICH: That’s a very common pattern to integrate liquid biopsy up front. It’s reliable, fast, and obviously in accordance here with guidelines to check for all these molecular markers. They would probably favor a different sequence of liquid vs tissue, but liquid biopsy is so much more accessible than tissue biopsy that I think the reality overtook the guidelines. Everybody [is] testing KRAS with these broad panels, then the PD-L1 testing in a second step, and everybody is using next-generation sequencing [NGS] already.

DIETRICH: When you have plasma testing up front, you probably would already have information to the full extent. [Is] anybody rebiopsying for tissue biopsy if there’s an insufficient quantity?
AZZI: Yes, if the liquid biopsy results are not convincing, [if] there is anything that’s not detectable, and if the patient is a nonsmoker, I would go for a rebiopsy. [If] they’re very symptomatic, then I do chemotherapy alone without immunotherapy, then do the rebiopsy.
DIETRICH: That’s a very reasonable path. It just takes so much time to repeat it and get the tissues resent. Do you still run into the 14-day rule, [where] hospitals don’t want to send out these tissues for analysis?
AZZI: Yes, of course. For Medicare patients who are diagnosed as inpatient, then yes. It might be more time efficient if they’re willing to rebiopsy them, especially if they were in another institution that’s not going to release the tissue until 14 days. [In that case], it will be faster to rebiopsy, then send from your own institution.
DIETRICH: Have you identified any KRAS G12C mutations yet?
AZZI: Yes, I have a lot of them, but nobody has progressed so far from my cohort, so that’s good.
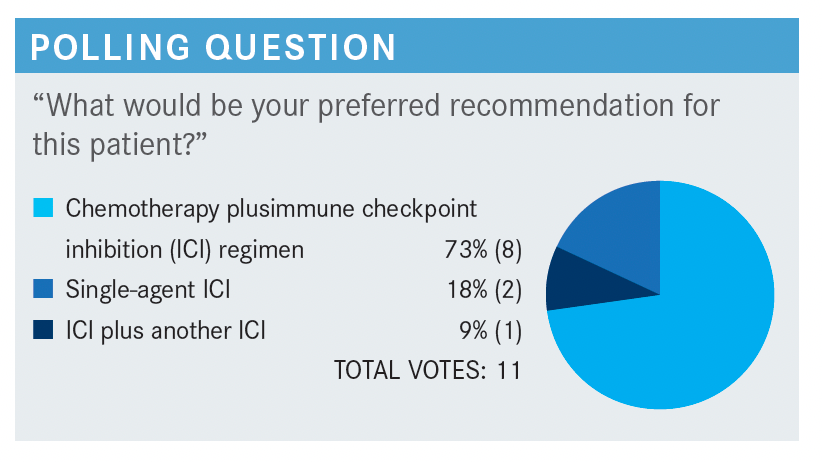
RAEZ: I think all of us are chemotherapy-friendly by training, so we like a strong overall response rate [ORR], and the combo gives a higher ORR. All the combos are 60% to 70%, whereas the single-agent immunotherapy is usually only a response rate of 40%.
That doesn’t mean the combos have a better survival, but most of us prefer first to secure a good response if the patient tolerates [the treatment] because the combo is more toxic than single agent.
DEZARRAGA: I also chose the combination of chemo and immunotherapy for those reasons. You mentioned the patient was symptomatic, so we want to use immunotherapy, but we also want to get a quicker and higher chance of responding. That’s why I chose [No. 1].
DIETRICH: Is the person who voted for the combination immunotherapy willing to comment on their choice of medication?
SHARMA: I was tempted because there is a 2-cm single solitary brain mass, so this would be 1 patient I would at least consider ipilimumab [Yervoy] plus nivolumab [Opdivo] plus maybe 4 cycles of chemotherapy there, or just give ipilimumab plus nivolumab. I know there’s a lot of tumor burden there, too, [so I would also try not] to give radiation up front.
DIETRICH: If I look at the American Society of Clinical Oncology data from this year, for single-agent ICIs, it’s
clear that the closer you get to 100%, you have an incremental benefit with each 10% that you’re increasing.1 I voted for the addition of chemotherapy up front as well, because I think the patient was symptomatic enough and had large enough tumor burden that I’d be concerned about it.
For these isolated brain metastases, we use radiation therapy more and more generously, but that’s more like a practice pattern rather than an evidence-based approach. They’re all valid here, but I think this would be a symptomatic patient case.
Edgardo S. Santos, MD

SANTOS: Basically, I would do that same thing. If the patient is symptomatic, I would look for a response quickly. In that case, regardless of the PD-L1 [status or whether he’s] a high expressor, I will work with the chemoimmunotherapy. [In a different scenario, if the patient is asymptomatic] or has stage IV disease, then I will go with the single agent.
DIETRICH: Looking at the first-line therapy, the single-agent pembrolizumab [Keytruda], carboplatin and pembrolizumab, atezolizumab [Tecentriq], or cemiplimab [Libtayo] here in the first-line setting as preferred regimens.
Then, with similar level of evidence but emphasized by the NCCN panel here, is ipilimumab plus nivolumab plus chemotherapy, then carboplatin, paclitaxel plus bevacizumab [Avastin] plus atezolizumab. Is anybody using the quadruplet in any setting?2
RAEZ: I don’t know. Maybe if you give a cisplatin plus pemetrexed [Alimta] adjuvant, then the patient fails 6 months later—I don’t think we should repeat the pemetrexed then.
DIETRICH: Anybody for EGFR or ALK mutations upon progression?
SANTOS: That was my standard of care before, but I changed my approach to those patients when [the patient progresses] on EGFR mutation, especially if the truncal mutation is still present.
DIETRICH: Do you continue the osimertinib [Tagrisso] when you start the quadruplet?
SANTOS: I will not use the quadruplet, but I will keep the osimertinib.
AZZI: When the patient progresses on an EGFR inhibitor you don’t do the quadruplet anymore? What do you do?
SANTOS: After reviewing everything and looking [at] all the data developing on antiangiogenetic plus EGFR mutation, the IMpower150 [NCT02366143] is driven by the addition of bevacizumab more than any other thing.3
With osimertinib, one of the beautiful things of that drug is protecting the patient [from] brain metastases or getting a great response in the brain. If the progression is below that and the brain is fine, I would not remove the osimertinib in my patients, especially if the truncal mutation remains. So I will add chemotherapy, but I will keep osimertinib on board.
DIETRICH: This is getting challenging to pick, and we’ve heard a lot of good arguments for each of these approaches. The brain metastases, the molecular selection, the clinical features—this is not going to be as straightforward as carboplatin/paclitaxel used to be for us, and [it’s] never going to be straightforward again. In the nondriver mutations, we still have a lot of prioritizing to do.
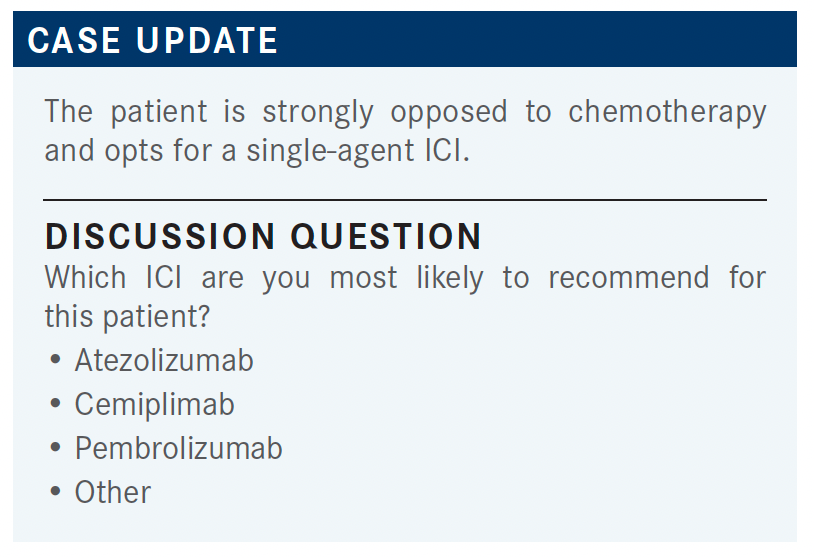
RAEZ: You can use any of the 3. We start with pembrolizumab, and probably the market share is pembrolizumab, followed by atezolizumab, and now cemiplimab.
DIETRICH: All of them are important. With efficacy, we’re seeing similar effects, but are there any differentiating factors in the trial design or in the data, the way that you would be including in the decision-making which one to choose? Do you have any formulary preferences? Is there anything that’s institutionally mandated in your case?
HURTADO: I basically take everything into consideration. I try to look at the patient, the goal of care, and then I select [treatment]. I’m more biased toward pembrolizumab, because I think there is a lot of data with it. Before we had nivolumab and other PD-L1 inhibitors that have a longer treatment interval at 4 weeks or 6 weeks, pembrolizumab was 3 weeks vs nivolumab, which was 2 weeks, for example.
CARTWRIGHT: I don’t think it makes much sense to switch from [one] checkpoint to another. I just use pembrolizumab in everybody. There [are] no head-to-head data, and it’s just confusing to the staff and everybody else to switch every time a patient walks in. Without head-to-head data, as you said before, it’s practically impossible to do cross-trial comparisons in these patients, so I just pick 1 and use it for most everybody.

AZZI: I don’t know [whether] you all noticed recently that NeoGenomics wants to switch away from using the 22C3 [test] to their own in-house assay. They were not going to stop running 22C3, and a lot of hospitals use that. So I thought, “How are they going to do that without any validation?”
DIETRICH: The 22C3 assay is kind of the standard. It would be hard for me to see why NeoGenomics—I’m not sure [whether] this is a technical issue or [whether] they’re using a different platform, but aside from the SP142 assay, they have a pretty good congruency. What do you use in your practice?
SANTOS: [I use the 22C3 assay], but when we have a long consultation, I send this to Caris or Tempus, someone that [has] DNA and RNA NGS. I don’t recall on Tempus, but on Caris, if it’s lung cancer, they will report [the 3 of them] to you.
They will report [to] you [the] 22C3, SP263, and SP142 assays. In that case…if I want to use pembrolizumab, my 22C3 assay is there. That’s why at least Caris does that. I don’t know about other vendors. I don’t remember correctly [whether] Tempus does that too.
RAEZ: The problem is the technology. Dako and Ventana run different machines, so it’s not easy for each hospital in [the US] to buy both technologies. For us, [this is just for lung cancer]. We only care about these 4 or 3 markers. But remember, there are indications for other cancers, then they do the immunohistochemistry for other things, even for TTF1.
That’s why it was a pain in the neck [before], because if you have the Dako machine, you can only run the Dako. [If] you have the Ventana, you can only run SP142. But now that we have validated, SP263 doesn’t matter. If you only have the Ventana machines, you can do SP263. If you have the Dako machines, you do the CD28. Now I think we’re fine. If you run SP263 instead of SP142, I think they are equivalent now, according to what we have done. So your hospital needs to buy only 1.
DIETRICH: The frontline indications here were all greater than 50% except for pembrolizumab.³ The 1 [to] 49 approval [is] controversial, and [it] probably shouldn’t be used alone. There were some differences with dosing: twice weekly over 3 and 4 weeks with atezolizumab, or 3 times weekly for cemiplimab, then 3 or 6 times weekly for pembrolizumab. I don’t want to open the box of when to stop. I’m not sure we have a safe end point here, and the warnings and precautions here [are] relatively similar.
So how long do you continue the single agent? It sometimes gets dictated by the circumstances. Two years in most trials, so that’s kind of a benchmark for most targets, below which we typically don’t stop. It’s sometimes also patient preference.
AZZI: Usually 2 years, but recently I’ve been using circulating tumor DNA to see [whether] they’re really negative at that time point before discontinuation. Because some of these patients are not, and they might be deriving that benefit. And if you stop them, they might progress, and you don’t know [whether] they really progressed on PD-L1, [whether they are] resistant, or [whether they are] progressing because you took them off. It’s hard to answer that question in a timely manner, but if you know what their circulating tumor DNA status is, that helps you monitor them longitudinally after discontinuation.
References:
1. Stenger M. Outcomes and toxicity with single-agent immune checkpoint inhibitor treatment in patients with cancer aged ≥ 80 years. The ASCO Post. November 11, 2021. Accessed November 24, 2021. https://bit.ly/3nPH897
2. NCCN. Clinical Practice Guidelines in oncology. Non-small cell lung cancer, version 7.2021. Accessed November 24, 2021. https://bit.ly/3HQ6bAW
3. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi:10.1016/S0140-6736(15)01281-7