Molecular Drivers of Cancer to Be Focus of Discussions at Breast Cancer Conferences
As the breast cancer setting quickly changes with each advancement, physicians who care for these patients will need to know how to apply these emerging treatments to the standards of care in their practice.
Joyce A. O Shaughnessy, MD

Joyce A. O Shaughnessy, MD
As the breast cancer setting quickly changes with each advancement, physicians who care for these patients will need to know how to apply these emerging treatments to the standards of care in their practice. It is proving to be an exciting, but dizzying time and that’s why Joyce A. O’Shaughnessy, MD, the Celebrating Women Chair in Breast Cancer Research at Baylor Charles A. Sammons Cancer Center and chair of the Breast Cancer Prevention Research Program at The US Oncology Network in Dallas, Texas, spoke with Targeted Therapies in Oncology. She offered an overview of the Miami Breast Cancer Conference® held by Physicians’ Education Resource, LLC (PER®) and other upcoming PER® breast conferences.
“At all the breast cancer conferences, novel therapeutics are focused on the molecular drivers of the cancer,” O’Shaughnessy said. “For every subtype of breast cancer, there are multiple agents being developed that do that. Those are always highlighted because they go hand in hand. Any data about the new agents can contribute to the understanding of the biology.” Another hot topic at the conferences will be advances in immunotherapy for breast cancer, added O’Shaughnessy.
O’Shaughnessy was a speaker for the 37th Annual Miami Breast Cancer Conference,® which was held in March and will be the program chair at the 19th Annual International Congress on the Future of Breast Cancer® East and the cochair at the19th Annual International Congress on the Future of Breast Cancer® West in July and August, respectively. She is also the activity chair of the 18th Annual School of Breast Oncology® conferencer in November.
She explained that the Miami conference is about making sure that physicians understand how to use the new treatments and methods, what the main adverse events of these therapies are, and how to optimize and sequence. The conference elucidates how these advances are adopted to improve the standard of care in this patient population. The conference also gives physicians a chance to debate areas of controversy to further deliver personalized care to the patient with breast cancer.
O’Shaughnessy was a speaker for the 37th Annual Miami Breast Cancer Conference,® which was held in March and will be the program chair at the 19th Annual International Congress on the Future of Breast Cancer® East and the cochair at the19th Annual International Congress on the Future of Breast Cancer® West in July and August, respectively. She is also the activity chair of the 18th Annual School of Breast Oncology® conferencer in November. She explained that the Miami conference is about making sure that physicians understand how to use the new treatments and methods, what the main adverse events of these therapies are, and how to optimize and sequence. The conference elucidates how these advances are adopted to improve the standard of care in this patient population. The conference also gives physicians a chance to debate areas of controversy to further deliver personalized care to the patient with breast cancer.
The overarching theme for all of these conferences is to help physicians learn how to apply the data in their practice. A key topic gaining attention is the role of locoregional control, and there has been a trend toward performing less surgery over time because radiation therapy is becoming more comprehensive for high-risk patient populations. Partial breast irradiation for patients with lower-risk disease has become more common than whole breast irradiation as well, and in general, radiation therapy and surgery are becoming more personalized, according to O’Shaughnessey.
“[Physicians would be] able to choose to perform a lumpectomy instead of a mastectomy because they have minimized the size of the tumor, and less axillary surgery, which is important,” she explained. “Taking out fewer axillary lymph nodes [is] decreasing the risk of lymphedema.”
One of the conferences that covers this is The School of Breast Oncology ®, a 3-day boot camp that O’Shaughnessy runs; she describes it as a “deep dive” into the breast cancer treatment. It covers “all the important literature in breast cancer, but [it’s still] practical.”
“It’s all case-based discussion for the surgical oncologist, medical oncologist, and radiation oncologist to come and give an in-depth update on all of the key data around [the] management strategies for breast cancer, including breast cancer prevention and genetic germline evaluation,” O’Shaughnessy said. “What are the data around diet and exercise and taking care of the host, what about breast cancer in pregnancy, what about toxicity management; there’s a big emphasis on radiation and surgical issues.”
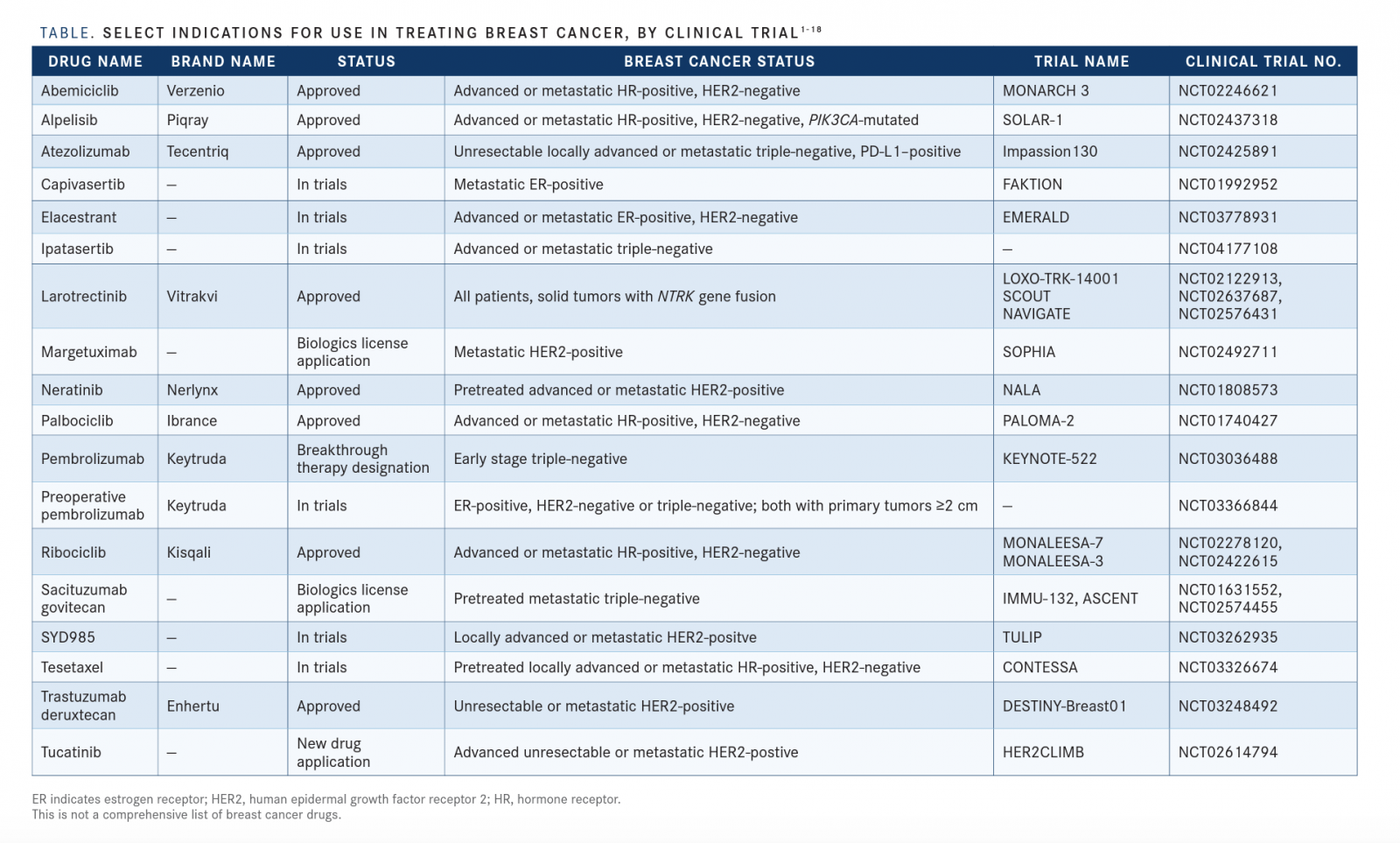
A major trend she has been seeing in the breast cancer setting is the increase of molecularly based therapies that target the driving pathogenesis of the cancer, such as alpelisib (Piqray). It is a PI3 kinase α-specific inhibitor approved by the FDA for patients with estrogen receptor (ER)-positive breast cancer andPIK3CAmutations[TABLE]. With the approval of alpelisib, there is a reason for patients with ER-positive disease to undergo next-generation sequencing, liquid biopsies, and blood tests for circulating tumor DNA to find any mutations.
“As I look over the years I’ve been in the breast cancer world, [it’s been] increasingly focused on targeting…cancer with increased biologic understanding of what the key drivers of the breast cancer are at that time,” O’Shaughnessy said.
She feels that oncology professionals would benefit from the conferences because of how quickly new agents are being discovered and receiving, or waiting on, FDA approval. As for a drug still waiting on the FDA’s approval, she discussed the case of tucatinib, which showed positive data in patients with brain metastases and improved survival in those with HER2-positive breast cancer at the San Antonio Breast Cancer Symposium. Because it is not FDA approved yet, caregivers need to know its efficacy so they can use it for their patients if needed. These conferences are helpful to bridge this gap in knowledge.
“[Physicians need] to know which patients it will be useful for and what the data showed,” O’Shaughnessy said. “Because there’s so many new data that you can apply tomorrow in your practice, you’ve got to know who and how to do that.”
“And then there are so many new agents coming along quickly, that by the time they get to the next meeting, the agents will already be here,” she continued. “[Physicians] have to learn about them in advance because the FDA approvals are coming quickly. It’s the practical optimal management of your patient Monday morning.
”For practicing physicians, keeping up with the current landscape can be challenging without conferences such as those held by PER®. Providing practical information regarding how to use treatments to those taking care of patients with breast cancer is a necessity.
Another big topic has been checkpoint inhibition, which have shown exciting data in the curative setting for patients with triple-negative breast cancer, said O’Shaughnessy. For instance, atezolizumab has recently been approved in the triple-negative space for patients who have PD-L1-positive status.
“What we’re waiting for is some of the adjuvant CDK4/6 inhibitor trials to report their progress in patients with high-risk ER-positive breast cancer, such as the PENELOPE-B trial [NCT01864746] which we should get the data on later this year.”
In this trial, patients received standard endocrine therapy and were randomized to 1 year of palbociclib [Ibrance] versus placebo after treatment with neoadjuvant chemotherapy and surgery. That trial and others for CDK4/6 inhibitors in the adjuvant setting will report within the next year, O’Shaughnessy hopes. These trials could lead to the FDA approval of different CDK4/6 inhibitors in the curative setting.
Going over these kinds of therapies, sequencing options, and new standards of care are the prime goals of the PER® breast cancer conferences; physicians who attend will be brought up to speed on how to treat the next patient they see.
“All those [conference objectives] are to take the new data that’s been presented already and help doctors understand how to use it in their practice,” O’Shaughnessy said.
References
- FDA approves abemaciclib as initial therapy for HR-positive, HER2-negative metastatic breast cancer. FDA website. https://bit.ly/2J2E4kX. Published February 26, 2018. Accessed March 20, 2020.
- FDA approves alpelisib metastatic breast cancer. FDA website. https://bit.ly/33xs0BB. Updated May 28, 2019. Accessed March 20, 2020.
- FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast cancer. FDA website. https://bit.ly/33xs0BB. Updated March 18, 2019. Accessed March 20, 2020.
- Jones RH, Casbard A, Carucci M, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial.Lancet Oncol. 2020;21(3):345-357. doi: 10.1016/S1470-2045(19)30817-4
- Bardia A, Aftimos P, Bihani T, et al. EMERALD: Phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol. 2019;15(28):3209-3218. doi: 10.2217/fon-2019-0370
- Roche’s ipatasertib in combination with Tecentriq and chemotherapy shows promising anti-tumour activity in triple-negative breast cancer in early phase trial. Roche website. https://bit.ly/396quHQ. Published April 1, 2019. Accessed March 20, 2020.
- FDA approves Larotrectinib for solid tumors with NTRK gene fusions. FDA website. https://bit.ly/2Ws8SU2. Updated December 14, 2019. Accessed March 20, 2020.
- MacroGenics Announces Submission of Margetuximab Biologics License Application to U.S. FDA [press release]. Rockville, Maryland: MacroGenics, Inc; Decmeber 19, 2019. https://bit.ly/2PIDehm. Accessed March 20, 2020.
- FDA approves neratinib for metastatic HER2-positive breast cancer. FDA website. https://bit.ly/394gD57. Updated February 26, 2019. Accessed March 20, 2020.
- Palbociclib (IBRANCE). FDA website. https://bit.ly/2QBZv0m. Published March 31, 2017. Accessed March 20, 2020.
- Merck’s KEYTRUDA® (pembrolizumab) Plus Chemotherapy Showed Statistically Significant Increase in Pathological Complete Response Versus Chemotherapy as Neoadjuvant Therapy in Early-Stage Triple-Negative Breast Cancer (TNBC). Merck website. https://bit.ly/2Qty1tJ. Published September 29, 2019. Accessed March 20, 2020.
- McArthur HL, Basho R, Shiao SL, et al. Abstract P2-09-07: preoperative pembrolizumab (Pembro) with radiation therapy (RT) in patients with operable triple-negative breast cancer (TNBC). Can Res. 2019;79(suppl 4; abstr P2-09-07). doi: 10.1158/1538-7445.SABCS18-P2-09-07.
- FDA expands Ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer. FDA website. https://bit.ly/397mF4X. Published July 18, 2018. Accessed March 20, 2020.
- Immunomedics Announces FDA Acceptance for Filing of Biologics License Application Resubmission for Sacituzumab Govitecan to Treat Metastatic Triple-Negative Breast Cancer. Immunomedics, Inc. Published December 26, 2019. https://bit.ly/37iQ4ZD. Accessed March 20, 2020.
- [vic-]trastuzumab duocarmazine (SYD985). Synthon Biopharmaceuticals website. https://bit.ly/3aaZBUj. Accessed March 20, 2020.
- O’Shaughnessy J, Piccart M, Schwartzberg LS, et al. CONTESSA: A multinational, multicenter, randomized, phase III registration study of tesetaxel plus a reduced dose of capecitabine in patients (pts) with HER2-, hormone receptor + (HR+)
