Managing Symptoms of Diarrhea Improves Medication Adherence in Patients Treated With Abemaciclib
Dose escalation may be beneficial for patients starting treatment regimens with a high incidence of toxicities that may lead to abrupt patient self-discontinuation.
CDK4/6 inhibitor abemaciclib (Verzenio) was initially approved for the treatment of hormone receptor (HR)–positive, HER2-negative advanced or metastatic breast cancer by the FDA in 2017 both as monotherapy and in combination with fulvestrant. As a monotherapy, the kinase inhibitor was approved for patients who had previously received endocrine therapy and chemotherapy, and as a combination, it was approved in patients with disease progression following endocrine therapy.1
The monotherapy indication was based on results from the single-arm phase II MONARCH 1 trial (NCT02102490) in which the median progression-free survival (PFS) in this patient population was 6 months (95% CI, 4.2-7.5) and the median overall survival (OS) was 17.7 months (95% CI, 16.0-not reached).2 The combination approval was based on results from the phase III MONARCH 2 trial (NCT02102490), in which investigators reported that adding abemaciclib to fulvestrant reduced the risk of disease progression or death by 45% versus fulvestrant alone.3
The MONARCH 1 trial included 132 patients with HR-positive/HER2-negative metastatic breast cancer who progressed during or after endocrine therapy and chemotherapy. The median age was 58 years (range, 36-89), and 44.7% of patients had an ECOG performance status of 1, 90.2% had visceral disease, and 85.6% had at least 2 metastatic sites.
Objective response rate (ORR) was the primary outcome measure. Secondary end points included duration of response (DOR), PFS, OS, clinical benefit rate, and safety.
The investigator-assessed, confirmed ORR was 19.7% (n = 26; 95% CI, 13.3%-27.5%), which included all partial responses (PRs). The proportion of patients with stable disease (SD) ≥6 months was 22.7%, leading to a clinical benefit rate (complete response [CR] + PR + SD ≥6 months) of 42.4%. The median time to response was 3.7 months, and the median DOR was 8.6 months. Thirty-four patients had progressive disease.
The most common nonlaboratory, all-grade adverse events (AEs) were diarrhea (90.2%), fatigue (65.2%), nausea (64.4%), decreased appetite(45.5%), and abdominal pain (38.6%). The grade 3 rates of these events were 19.7% for diarrhea, 12.9% for fatigue, 4.5% for nausea, 3.0%fordecreased appetite, and 2.3% for abdominal pain.
Leukopenia (27.7%) and neutropenia (22.3%) were the most common grade 3 laboratory AEs. The only grade 4 AE of any kind in the trial was neutropenia, which occurred in 4.6% of patients.
Serious AEs occurred in 24.2% (n = 32) of patients, with AEs leading to treatment discontinuation in 7.6% (n = 10) of patients. Dose reductions were required for 49.2% (n = 65) of patients. The most common reasons for dose reductions were diarrhea (20.5%) and neutropenia (10.6%). There were 2 patient deaths during treatment and 1 patient death within 30 days after study discontinuation.
In the MONARCH 2 trial, 669 patients wererandomized in a 2:1 ratio to abemaciclib plus fulvestrant (n = 446) or fulvestrant plus placebo (n = 223).2 Patients had progressed during neoadjuvant or adjuvantendocrine therapy, within 12 months of adjuvant endocrine therapy, or during frontline endocrine treatment for metastatic disease.
Following 379 PFS events in the intent-to-treat population, the median PFS was 16.4 months in the abemaciclib arm versus 9.3 months in the fulvestrant-alone arm (HR, 0.553; 95%CI, 0.449-0.681; P<.001).The ORRs among patients with measurable disease were 48.1% and 21.3% in the abemaciclib and control arms, respectively.
The 48.1% ORR in the abemaciclib cohort for patients with measurable disease included a CR rate of 3.5%. There were no CRs in the control arm. The median DOR was 25.6 months in the placebo arm and had not yet been reached in the fulvestrant arm.
The most common all-grade treatment-related AEs with the abemaciclib combination versus fulvestrant alone were diarrhea (86.4% vs 24.7%), neutropenia (46.0% vs 4.0%), nausea (45.1% vs 22.9%), and fatigue (39.9% vs 26.9%).
Themost frequently reported grade 3 AEs inthe abemaciclib versus fulvestrant-alone arms were neutropenia (23.6% vs 1.3%) and diarrhea (13.4% vs 0.4%). Grade 4 neutropenia occurred in 2.9% versus 0.4% of the patients in the abemaciclib and fulvestrant-alone groups, respectively. There were 3 deaths in the abemaciclib arm linked to treatment-related AEs compared withnone in the control arm.
Managing the Symptoms of Diarrhea
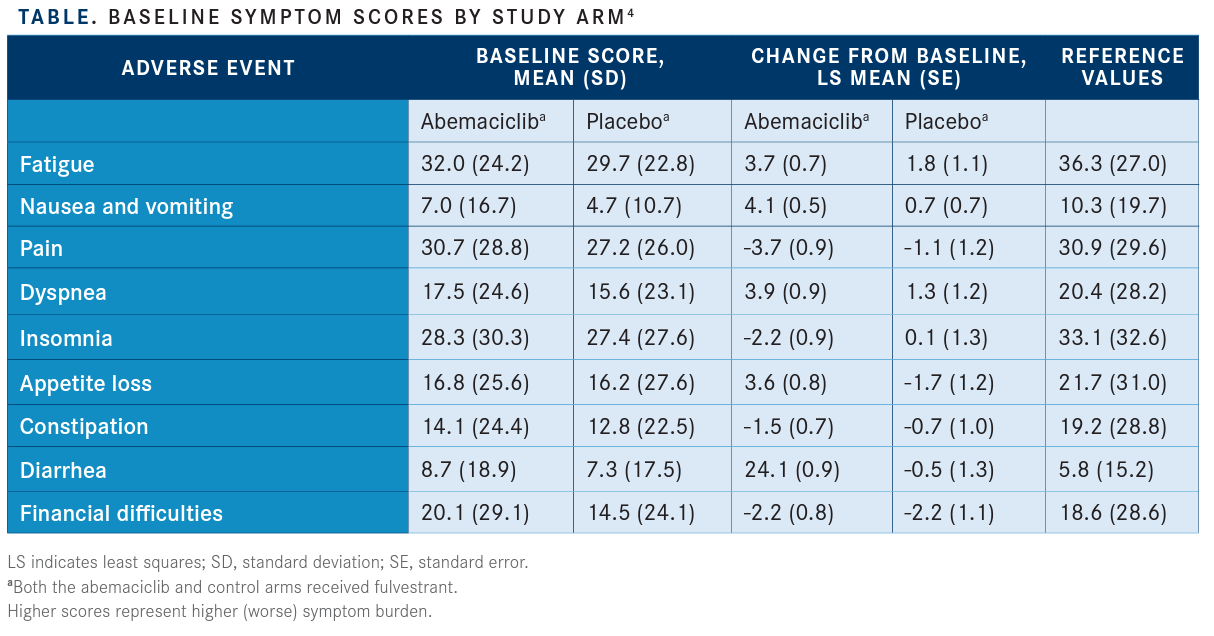
A recently published study by PeterA. Kaufman, MD,and colleagues evaluated pain, global health-related quality of life, functioning, and symptoms in patients who participated in MONARCH 2. The investigators reported that symptoms favored patients in the abemaciclib arm, with the exception of diarrhea, which favored the control arm(HR, 1.60; 95% CI, 1.20-2.10) (TABLE).
Pain and other quality-of-life symptoms were determined using the Brief Pain Inventory, ShortForm; European Organization for Research and Treatment of Cancer QoL Core 30 (QLQ-C30); and Breast Cancer Questionnaire (QLQ-BR23). Data were collected at baseline, cycle 2, every 2 cycles from cycles 3through 13, every 3 cycles thereafter, and 30 days post discontinuation.
Patients in the abemaciclib arm had a 4.9-month delay in pain deterioration compared with the control arm and a significantly greater time to sustained deterioration (TTSD) (HR, 0.76; 95% CI, 0.59-0.98) and a significantly higher score on the QLQ-C30 pain item (HR, 0.62; 95% CI, 0.48-0.79). TTSD for functioningand most symptoms, including fatigue, nausea and vomiting, and cognitive and social functioning, significantly favored theabemaciclib arm.
In general, diarrhea represents a particularly difficult AE to manage and contributes significantly to patient adherence.
Case Description
Physicians from Tennessee Oncology in Nashville, Tennessee, described a patient with de novo stage IV, estrogen receptor–positive, progesterone receptor-positive, HER2-negative breast cancer. She developed pleural metastasis in September 2014. She was subsequently started on letrozole in September 2014, which she tolerated well. In June 2018, it was determined that the patient had developed low-volume progressive disease, and she was switched to exemestane. New hepatic metastases were identified in February 2019, with clear progressive disease. At this time, she was started on endocrine therapy plus abemaciclib.
Patient Care
To preemptively limit potential AEs and improve adherence, we implemented 2 key approaches with this patient.
First, due to concerns about treatment tolerability and quality of life, the patient was started on a reduced dose of abemaciclib at 100 mg twice daily. Although there are no specific data for this approach for patients who undergo treatment with abemaciclib, this has been studied in other medications that have a propensity for diarrhea, such as regorafenib (Stivarga)5 and neratinib (Nerlynx).6
Second, we used proactive, instead of reactive, communication to help educate the patient and prophylactically manage AEs. Once the medication was ordered in our electronic health record system, the medication along with written information and an AE management kit was sent to the patient’s home. When our specialty pharmacy received confirmation of medication receipt, we allowed an hour for the patient to review the provided information and develop her own questions.
Our patient education team then called the patient to discuss medication dosing, possible AEs, and strategies for AE management. For abemaciclib, the AE management kit provided includes a 30-day supply of loperamide with instructions on proper use. Our instructions state to not use Imodium prophylactically but to begin use after the first episode of diarrhea. Specifically, the provided instructions direct the user to take 2 4-mg capsules after the first episode of diarrhea, followed by 1 capsule for eachsubsequent loosestool, witha maximum of 8 capsules in a 24-hour period. Finally, the patient was also instructed to contact us if she experienced more than 4 loose stools per day so that we could increase the aggressiveness of antidiarrhea management before further issues arose.
After the initial education phone call, our pharmacy team reached out to the patient again on day 7 of her first cycle of abemaciclib to assess tolerability and adherence, which she reported were both excellent. The purpose of this second call was to assess tolerability, reinforce AE management strategies, and promote adherence.
We scheduled her return visit to the clinic 14 days after treatment initiation. At that time, she denied any shortness of breath, nausea, or abdominal pain. However, she did report some mildepisodesof diarrheaand was reeducated on the proper use of antidiarrheal agents and the importance of maintaining hydration. Her diarrhea remained controlled for the remainder of cycle 1 and the subsequent cycle 2 as well.
Becausethe patient was tolerating the 100-mg abemaciclib dose well, the dose was then escalated to 150 mg twice a day starting with cycle 3. This conservative approach helped the patient successfully maintain medication adherence during the most vulnerabletime for her to experience AEs that may have possibly led to dose interruptions or discontinuation. She continued to show excellent adherence and tolerance to the 150-mg twice-daily dose while using loperamide as needed for episodes of diarrhea. Throughout her treatment, she continued to have an excellent response to treatment, as demonstrated in her scans as well as carcinoembryonic antigen and cancer antigen 15.3 results. However, in her last months of abemaciclib treatment her performance status started to decline, and treatment was ultimately discontinued.
In the MONARCH 2 trial, diarrhea occurred in over 85% of patients receiving abemaciclib. The median time to onset of diarrhea was6 days. Additionally, the incidence of grade 2/3 diarrhea was markedly increased in the first month of treatment but gradually decreased as patients continued treatment with abemaciclib. During the study, the incidenceand severity of diarrhea led to over 20% of patients requiring dose reductions.3
Similar approaches to managing medications with a high incidence of particular AEs, mainly diarrhea, have been studied in other medications such as regorafenib and neratinib. The ReDOS trial evaluated dose escalation of regorafenib; the primary endpoint was the proportion of patients able to complete 2 cycles of therapy and initiate cycle 3. The study showed that patients on the dose-escalation regimen had a more tolerable AE profile and were more likely to complete the first 2 cycles of regorafenib compared with the control arm.5
The results of this trial ultimately led the National Comprehensive Cancer Network to includethis dosingstrategyintheir guidelines.7
The CONTROL trial with neratinib is ongoing, but preliminary results show fewer medication discontinuations due to diarrhea even when compared with regimens containing diarrhea prophylaxis. There are currently no clinical trials studying this dose-escalation strategyin abemaciclib; however, our case example above suggests utility in certain populations with a high risk of drug toxicity.6
Dose escalation may be beneficial for patients starting treatment regimens with a high incidence of toxicities that may lead to abruptpatientself-discontinuation. Patient factors may also contribute to decisions about thebest AE management strategies, as some patients may be more sensitive to AEs and thus quicker to stop taking their medication. Personalized dosing strategies, proactive symptom management techniques, patient education, and appropriately timed patient follow-ups are all necessary to ensure that patients are put in the best position to be successful on treatment.
References:
1. FDA approves abemaciclib for HR-positive, HER2-negative breast cancer [news release]. Silver Spring, MD; Office of Communications; September 28, 2017. bit.ly/3dugoUu. Accessed March 25, 2020.
2. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer [published correction appears inClin Cancer Res. 2018;24(21):5485]. Clin Cancer Res.2017;23(17):5218-5224.doi:10.1158/1078-0432.CCR17-0754.
3. Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy.J Clin Oncol. 2017;35(25):2875-2884. doi: 10.1200/JCO.2017.73.7585.
4. Kaufman PA, Toi M, Neven P, et al. Health-related quality of life in MONARCH 2: abemaciclib plus fulvestrant in hormone receptor-positive, HER2-negative advanced breast cancer after endocrine therapy.Oncologist. 2020;25(2):e243-e251. doi: 10.1634/theoncologist.2019-0551.
5. Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study.Lancet Oncol. 2019;20(8):1070-1082. doi: 10.1016/S1470-2045(19)30272-4.
6. Chan A, Hurvitz SA, Marx G, et al; CONTROL Investigators. Effect of prophylaxis or neratinib dose escalation on neratinib-associated diarrhea and tolerability in patients with HER2-positive early-stage breast cancer: phase II CONTROL trial.Cancer Res. 2020;80(suppl 4; abstr P5-14-03). doi: 10.1158/1538-7445.SABCS19-P5-14-03.
7. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Breast Cancer (version 3.2020). bit.ly/2RmOVe1. Accessed March 25, 2020.
