HITTING THE TARGET: Multigene Tests Gain Foothold in More Clinical Settings
Broad adoption of testing methods that allow for assessing all possible actionable targets and diagnostic markers in advanced cancer is becoming more complex with greater availability of lifesaving targeted therapies.
John Simmons, PhD

In the treatment of many advanced cancers, molecular profiling for the detection of tumor mutations and genomic signatures is recognized as the standard of care. But broad adoption of testing methods that allow for assessing all possible actionable targets and diagnostic markers becomes more complex with greater availability of lifesaving targeted therapies.
To keep up with the momentum in the development of precision oncology agents, multigene testing assays have come into focus in certain solid tumors, such as non–small cell lung cancer (NSCLC),1 and with wider availability, these broader testing methods are making headway in the treatment of patients in more clinical settings.
The main disadvantage of traditional companion diagnostics lies in the restriction of testing to 1 bio-marker per drug per tumor. Diseases with multiple potential targets require broader testing, and the growing prevalence of tumor-agnostic drug indica-tions continues to expand this list.2
“As many as a third of patients who might be eligible for targeted therapy are missing that therapy…when so much work has been done to bring these great pre-cision therapies to the marketplace,” John Simmons, PhD, vice president of Translational Medicine at Per-sonal Genome Diagnostics, a biotechnology company in Baltimore, Maryland, said in an interview with Targeted Therapies in Oncology (TTO).
For example, in the case of NSCLC, the National Comprehensive Cancer Network (NCCN) guide-lines recommend testing for aberrations in EGFR, ALK, ROS1, and BRAF in all patients with adeno- carcinoma histology.
The guidelines also note that testing of all 4 genes may be considered in patients with squamous cell carcinoma whose biopsies show mixed histology, and testing of EGFR and ALK may be considered for never- smokers with squamous cell carcinoma.1 Added to this, the presence of KRAS mutations in patients with activating EGFR mutations predicts a poor response to anti-EGFR therapy,2 whereas the presence of an NTRK fusion indicates a positive response to TRK inhibitor therapy.1
Similar effects are seen in other tumor types. The coexistence of tumor EGFR expression and an NRAS or KRAS mutation in colorectal cancer denotes resis-tance to anti-EGFR therapy. Likewise, a mutation in the ABL kinase domain of BCR-ABL fusion–positive chronic myeloid leukemia conveys poor response to imatinib (Gleevec).2
Popular gene testing methods used in commercially available cancer assays today include polymerase chain reaction (PCR), for identifying a specific known mutation in a sample and next-genera-tion sequencing (NGS), for detecting many cancer-as-sociated mutations in a single patient sample.
According to a 2019 market analysis, PCR testing accounted for the largest share of the companion diagnostics market, likely because of the method’s ease of use and the widespread availability of kits and reagents.3 In the same report, the authors esti-mated that the fastest-growing segment in the market through 2024 would be NGS, with higher demand attributed to its improved sensitivity and specificity compared with traditional sequencing technologies, as well as faster identification of genetic diseases in a specific population or tissue type.4
Current Use in Practice
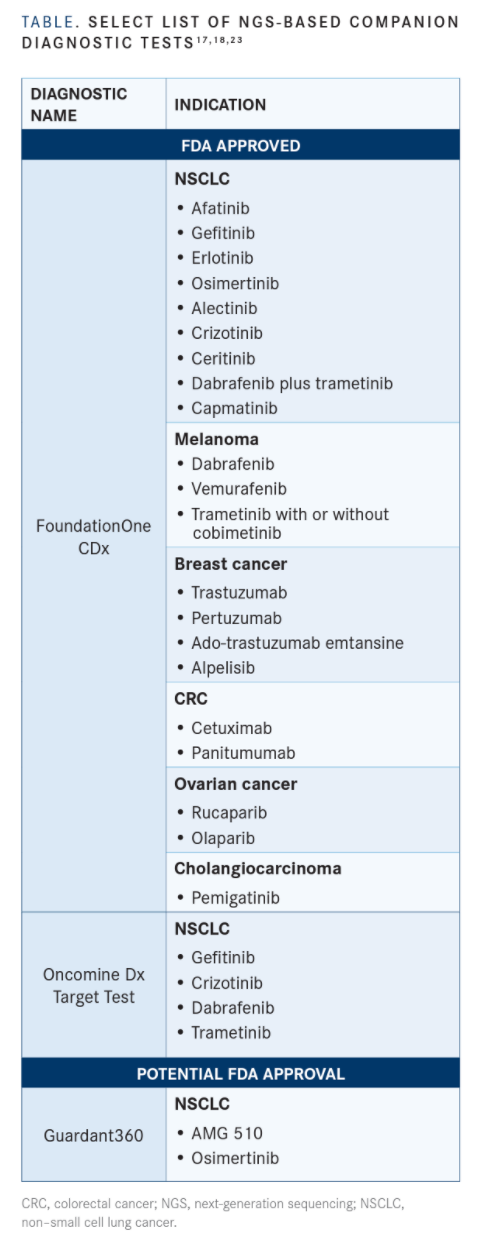
In 2017, the FDA granted premarket approval to the first NGS companion diagnostic test that could simultaneously test tumors for biomarkers associated with multiple NSCLC therapies. The Thermo Fisher Scientific Oncomine Dx Target Test evaluates 23 NSCLC-related genes, 3 of which could be used to identify patients eligible to receive therapy with gefitinib (Iressa), crizotinib (Xalkori), and the combination of dabrafenib (Tafinlar) and trametinib (Mekinist). The ability to test multiple genes at a time with this assay has also reduced the time it takes to match patients to therapy, which when done sequentially could take weeks rather than days.5
Other NGS tests have received approval from the FDA, mainly in the setting of treating advanced cancer, such as the FoundationOne CDx (F1CDx) test and MSK-IMPACT, an assay performed only in the diagnostic molecular pathology laboratories at Memorial Sloan Kettering Cancer Center.6,7
In the age of numerous viable targets in the treatment of different advanced cancers, these tests are being touted by leaders in the f ield. “NGS is referenced currently in 11 NCCN guidelines,” Simmons said. “Of the biomarkers that are reported, there are 60 that fall into the classification for having evidence of utility.”
Since initial approval of F1CDx for the detection of genetic aberrations in patients with NSCLC, melanoma, breast cancer, colorectal cancer, or ovarian cancer who may benefit from 1 of 15 FDA-approved therapies, additional indications for use have been added as new targeted therapies have become available. The test is capable of detecting substitutions, insertions and deletions, and copy-number alterations in a total of 324 genes, as well as select gene rearrangements, in DNA from tumor tissue specimens.8
In April of this year, another tumor type, cholangiocarcinoma, was added to the F1CDx label following approval of pemigatinib (Pemazyre) for the treatment of patients with metastatic cholangiocarcinoma harboring FGFR2 rearrangements.9,10
A month later, the test received another approval, this time for aiding in identification of patients with NSCLC who may be treated with capmatinib (Tabrecta), which is intended for use in those whose tumors have an MET exon 14 skipping mutation. This subset of patients represents 3% to 4% of patients with NSCLC.11,12
Refined Sequencing Methods Have Broader Reach
As NGS technology matures, more sophisticated methods are being developed, allowing more and more clinicians access to comprehensive genomic profiling tools. This gives them the ability to offer their patients the best possible therapy based on the presence or absence of biomarkers.
In April 2020, the FDA granted market clearance to PGDx elio tissue complete, a comprehensive, NGS-based tumor-profiling diagnostic kit that can be used in molecular diagnostics to detect single-nucleotide variants, insertions, and deletions in more than 500 genes, as well as copy-number alterations, translocations, microsatellite instability, and tumor mutation burden.13
“This test is cleared as a kit and software solution that can be run in any molecular pathology lab in the United States,” Simmons said. “Other kits that have gone through the FDA are just much smaller in their breadth of what they [can detect].”
The software that accompanies PGDx elio tissue complete was developed using machine learning to identify high-confidence somatic mutations without the need for a genomic analyst to go through the data, Simmons said. Using a specialized random forest classification model to evaluate a set of decision trees, a confidence score for each candidate variant is generated. This machine learning method was trained using a set of more than 30,000 somatic variants as well as a real-world representative sample of more than 2 million NGS errors and artifacts that might be falsely detected as variants.14
“It doesn’t require any further checking for accuracy by a human at the end, and that’s a big distinguishing feature for the test,” Simmons said. “Building that algorithm as part of our variant calling via the software that’s included in this device was incredibly important in ensuring that a patient, no matter whether they are being tested—in Baltimore, San Francisco, or even Beijing or Berlin—[would] get the same answer from the report.”
When asked about how this technology applies to physicians treating patients with advanced cancer, Simmons said, “It provides information that can guide clinical management, informs the use of targeted therapies and immunotherapies based on the results of the test, and identifies opportunities for clinical trial participation. It goes a long way in helping close that gap [between guideline recommendations and real-world care] because it can be integrated within the existing health care ecosystem.”
Approval to market the PGDx elio tissue complete was granted by the FDA’s Center for Devices and Radiological Health in response to a premarket notification (510[k]) filed by Personal Genome Diagnostics. By comparing PGDx elio tissue complete to a similar test that had already received marketing approval (MSK-IMPACT), the FDA determined that the 2 assays were “substantially equivalent” in terms of safety and efficacy. Therefore, the PGDx test could be sold without a premarket approval application.15
Plasma NGS Stands Out Another NGS assay being explored as a companion diagnostic for use with existing and novel targeted therapies is the Guardant360 test, which received expedited access pathway designation for breakthrough devices from the FDA in 2018.16 Since then, Guardant Health has partnered with pharmaceutical companies such as AstraZeneca and Amgen in support of commercialization of the assay as a companion diagnostic for targeted therapies.17,18
“FDA approval for some tests would be the natural next step and should be coming in the future,” Becky Nagy, MS, LGC, vice president of Medical Affairs at Guardant Health, a biotechnology company in Redwood City, California, said in an interview with TTO. “In terms [of] future utility, I see it expanding within the oncology space across all stages of cancer.”
Guardant360 is a plasma-based sequencing assay capable of detecting single-nucleotide variants, insertions and deletions, amplifications, and fusions in more than 70 genes, including PIK3CA, for which targeted therapy for breast cancer is approved.19
One study (NCT01870505) examining mechanisms of resistance to the PI3K inhibitor alpelisib (Piqray) in 38 patients employed plasma-based NGS testing using Guardant360, which demonstrated high concordance (89%) with tumor tissue testing for identifying PIK3CA mutations. The study results also demonstrated that PTEN loss-of-function alterations, which confer alpelisib resistance, cooccurred with PIK3CA mutations in a meaningful proportion of patients. The investigators concluded that before commencing treatment with alpelisib, patients would benefit from comprehensive genomic profiling (rather than testing PIK3CA alone) to identify resistance-associated alterations such as PTEN loss that would contraindicate PI3K inhibition.20
In addition, Nagy pointed out that Guardant360 can detect point mutations not included in the list of “hotspot” mutations identified by FDA-approved companion diagnostics, such as the therascreen PIK3CA RGQ PCR Kit. These non-hotspot variants include PIK3CA N345K and E453Q, which were seen in patients treated on the study.20-22
If approved, Guardant360 will be the first commercially available liquid biopsy with the FDA's acceptance.16
Looking Ahead
In addition to the abovementioned assays (TABLE),17,18,23 other tests have the potential to further enrich the precision oncology ecosystem.
In May 2019, the FDA granted Breakthrough Device designation to MI Transcriptome, an NGS-based in vitro companion diagnostic for detecting genetic alterations in tissue samples. The designation was for the detection of novel FGFR biomarkers, including gene fusions, in solid tumors.24 In April of this year, Caris Life Sciences, the company that developed the assay, submitted a Premarket Approval application to the FDA for the test.25
Also in 2019, Breakthrough Device designation was granted to a companion diagnostic assay developed by ArcherDX that is capable of analyzing more than 50 genes. The test is distinct from the others mentioned in that it can detect mutations in either circulating tumor DNA or a tumor sample.26
“[These tests] provide an opportunity for efficiency and for getting critical information for each and every patient, no matter where they are diagnosed. Patients in rural areas should have the same access to care as patients who are being treated at the top tertiary cancer centers in the big cities,” Simmons concluded. “Precision medicine isn’t precise without a test.”
References:
1. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Non-Small Cell lung Cancer (version 3.2020). Updated February 11, 2020. Accessed May 7, 2020. https://bit. ly/35KiGLQ
2. Khoury JD, Catenacci DV. Next-generation companion diagnostics: promises, challenges, and solutions. Arch Pathol Lab Med. 2015;139(1):11‐13. doi:10.5858/arpa.2014-0063-ED
3. Companion diagnostics market by product & service (assay, kit, software & service), technology (PCR, NGS, ISH, IHC), indication (breast, lung & colorectal cancer, cardiovascular disease), end-user (pharma companies, cro), region - global forecast to 2024. Published September 2019. Accessed May 7, 2020. https://bit.ly/3b8vfl2
4. Companion diagnostics market worth $7.3 billion by 2024. News release. MarketsandMarkets Inc; September 16, 2020. Accessed May 7, 2020. https://bit.ly/3cfvFaG
5. FDA approves first companion diagnostic test to simultaneously screen for multiple non-small cell lung cancer therapies. News release. Thermo Fisher Scientific; June 22, 2017. Accessed May 7, 2020. https://bit.ly/2yCgzxm
6. FDA grants marketing approval to FoundationOne CDx in vitro diagnostic. Updated December 1, 2017. Accessed May 7, 2020. https://bit.ly/2WaUhvG
7. MSK-IMPACT is the first tumor-profiling multiplex panel authorized by the FDA, setting a new pathway to market for future oncopanels. News release. Memorial Sloan Kettering Cancer Center; November 15, 2017. Accessed May 7, 2020. https://bit.ly/2Wbrkjd
8. Summary of safety and effectiveness data (SSED): FoundationOne CDx (F1CDx). April 17, 2020. Accessed May 7, 2020. https://bit. ly/2zlqcQS
9. Foundation Medicine receives FDA approval for FoundationOne CDx as the companion diagnostic for Pemazyre (pemigatinib), the first FDA-approved targeted therapy for adults with previously treated locally advanced or metastatic cholangiocarcinoma. News release. Foundation Medicine, Inc; April 17, 2020. Accessed May 7, 2020. https://bit.ly/3b8R9EF
10. FDA grants accelerated approval to pemigatinib for cholangiocarcinoma with an FGFR2 rearrangement or fusion. April 17, 2020. Accessed May 7, 2020. https://bit.ly/3frACix
11. Foundation Medicine receives FDA approval for FoundationOne CDx as the companion diagnostic for Tabrecta (capmatinib), the only FDA-approved MET inhibitor for patients with metastatic non–small cell lung cancer with METex14. News release. Foundation Medicine, Inc; May 6, 2020. Accessed May 7, 2020. https://bit.ly/3doZjdK
12. FDA grants accelerated approval to capmatinib for metastatic non–small cell lung cancer. May 6, 2020. Accessed May 7, 2020. https://bit.ly/3cdsFvj
13. Personal Genome Diagnostics receives FDA clearance for PGDx elio tissue complete, the first comprehensive genomic profiling diagnostic kit for oncology. News release. Personal Genome Diagnostics Inc; April 27, 2020. Accessed May 7, 2020. https://bwnews. pr/2ysYj9N
14. Wood DE, White JR, Georgiadis A, et al. A machine learning approach for somatic mutation discovery. Sci Transl Med. 2018;10(457):eaar7939. doi:10.1126/scitranslmed.aar7939
15. Indications for use – PGDx elio tissue complete. FDA. April 24, 2020. Accessed May 7, 2020. https://bit.ly/2LbqaOo
16. The Guardant360 assay receives expedited access pathway designation for breakthrough devices from FDA. News release. Guardant Health, Inc; February 15, 2018. Accessed May 7, 2020. prn. to/2yn0yLm
17. Guardant Health announces collaboration with Amgen to develop a global liquid biopsy companion diagnostic for AMG 510 KRAS G12C inhibitor. News release. Guardant Health, Inc; January 13, 2020. Accessed May 7, 2020. bit.ly/3eoHwoe
18. Guardant Health partners with AstraZeneca to develop blood-based companion diagnostic tests for Tagrisso and Imfinzi. News release. Guardant Health, Inc; December 13, 2018. Accessed May 7, 2020. bit. ly/3blS0TE
19. FDA approves alpelisib for metastatic breast cancer. May 24, 2019. Accessed May 7, 2020. https://bit.ly/2zhyIk0
20. Razavi P, Dickler MN, Shah PD, et al. Alterations in PTEN and ESR1 promote clinical resistance to alpelisib plus aromatase inhibitors [published online ahead of print March 23, 2020]. Nat Cancer. doi:10.1038/ s43018-020-0047-1
21. Summary of safety and effectiveness data (SSED): therascreen PIK3CA RGQ PCR Kit. FDA website. Published May 24, 2019. Accessed May 7, 2020. bit.ly/3cCYgXY.
22. Martínez-Sáez O, Chic N, Pascual T, et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 2020;22(1):45. doi:10.1186/s13058-020-01284-9
23. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). Food and Drug Administration. Accessed May 19, 2020. https://bit.ly/3e4Pdz9
24. Caris Life Sciences receives FDA Breakthrough Device designation for MI Transcriptome companion diagnostic test. News release. Caris Life Sciences; May 3, 2019. Accessed May 7, 2020. https://bit.ly/3bbng6U
25. Caris Life Sciences submits two PMA applications to the FDA for whole exome and whole transcriptome sequencing. News release. Caris Life Sciences; April 28, 2020. Accessed May 7, 2020. https://bit. ly/35HxZoq
26. ArcherDX’s companion diagnostic assay for both liquid biopsy and tissue specimens granted Breakthrough Device Designation by U.S. Food and Drug Administration. News release. ArcherDX, Inc; January 8, 2019. Accessed May 7, 2020. https://prn.to/2Wc8AQq

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More









