Addressing Skin-Related Toxicities Extends Positive Clinical Outcomes
Management of treatment-associated dermatologic adverse events that commonly occur as a result of cytotoxic or targeted therapy is critical to prevent dose modifications or interruptions that could negatively affect clinical outcomes.
Management of treatment-associated dermatologic adverse events (AEs) that commonly occur as a result of cytotoxic or targeted therapy is critical to prevent dose modifications or interruptions that could negatively affect clinical outcomes. According to Mario E. Lacouture, MD, who presented data related to the incidence of dermatologic AEs as part of the virtually held 2020 Community Oncology Conference, assuring patients who develop these toxicities that their symptoms are manageable may motivate them to institute strategies that do not affect their treatment dose.1
“About 25% of all patients with cancer receiving systemic therapy will develop dermatologic toxicities at some point during their [course of treatment],” Lacouture said. “About a third of cancer survivors will report that the dermatologic toxicities they experienced during their treatment [had a negative] impact.”
Although the reasons why are still unclear, many of the dermatologic toxicities discussed in the presentation have also been correlated with improved survival in certain tumor types. Lacouture said informing patients of this relationship could be helpful in comforting those who develop difficult toxicities. “The same pathways and proteins that are critical for malignant behavior are also important and critical for the homeostasis and functioning of the skin, hair, and nails,” he said.
Lacouture, who is director of the Oncodermatology Program at Memorial Sloan Kettering Cancer Center in New York, New York, added that there seems to be a discordance between diagnoses and the need for dose modifications observed between oncologists and dermatologists when patients present with dermatologic AEs, for which he hopes increased clinician education would help reduce.
“Dermatologic adverse events are usually class based and mechanism based. It is easy to determine what therapy a patient is receiving just by looking at their skin, and in some cases, hair or nails,” Lacouture said.
Skin Rash
Agents blocking the epidermal growth factor receptor (EGFR) have been strongly associated with dermatologic AEs, with about two-thirds of patients developing an acneiform rash that Lacouture said could have “disfiguring” effects on the face, chest, scalp, and upper back.
This AE is one of the most common reasons for dose modifications or interruptions, calls to the office, and unplanned visits that are caused by EGFR inhibitors (FIGURE).1
For first- and second-generation tyrosine kinase inhibitors as well as monoclonal antibodies inhibiting EGFR, prophylactic therapy is key for managing this dermatologic AE.
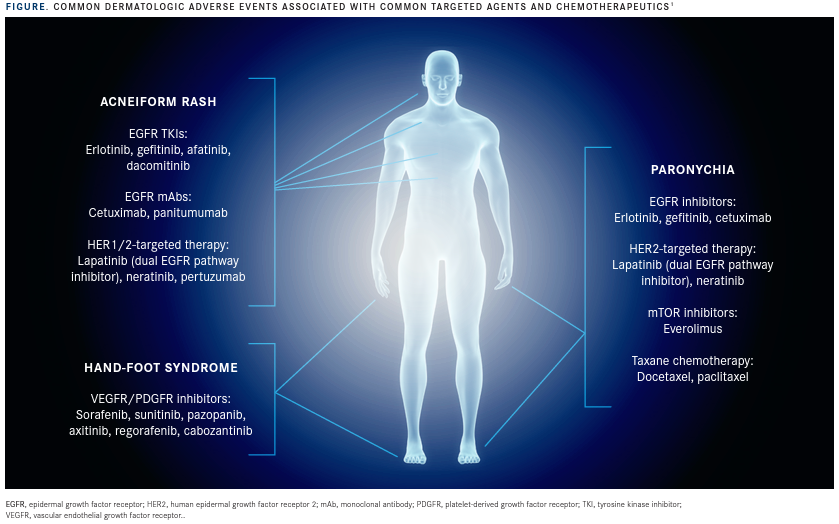
HER2 agents can also cause acneiform rash at a lower severity, which means a more reactive management strategy can be employed.
In the ARCHER 1042 (NCT01465802) phase 2 trial of patients treated with the EGFR inhibitor dacomitinib (Vizimpro), the addition of prophylactic oral doxycycline reduced the incidence of grade 2 or greater skin toxicity by over half as compared with a reactive management strategy in patients with non–small cell lung cancer (23.2% vs 46.6%). Applying topical steroids plus an oral probiotic was also an effective strategy for reducing the incidence of dermatologic AEs compared with placebo, but the benefits were less significant.2
Nail Toxicities
Another AE commonly occurring in patients treated with targeted agents is paronychia, a painful inflammation and infection around the lateral sides of the fingernails and toenails, arising in about 25% of patients treated with EGFR inhibitors. This manifestation of paronychia can also be seen in patients receiving HER2 or mTOR inhibitors, but at lower rates.
Paronychia under the nail plate can occur in patients receiving taxane chemotherapy. One retrospective study demonstrated that more than 80% of patients treated with docetaxel (n = 55) experienced some changes to their nails over the course of treatment, with up to 43% of patients reporting difficulties doing daily activities.3
Lacouture recommended that clinicians obtain a bacterial culture every time patients present with a discharging lesion because the incidence of gram-negative secondary infections is high, with about 40% of patients with paronychia developing gram-negative organisms at the site of infection. He recommended that AEs of grade 1/2 in severity be treated with over-the-counter topical antiseptics that patients can soak their nails in. AEs of grade 3 or higher can be managed with oral antibiotics based on sensitivity, with some patients requiring partial or full nail avulsion performed by a podiatrist or dermatologist.
To prevent taxane-induced nail changes, Lacouture said patients can have their hands and feet cooled during therapy administration based on findings from a study showing that the use of a frozen glove was effective at reducing negative effects on the nail bed. Comparing 1 gloved hand with an ungloved hand in all treated patients, all-grade events were observed at a rate of 51% versus 11%.4
Added to this, Lacoutre emphasized that the use of a cooling glove has positive effects that extend beyond relieving discomfort on the surface of the skin. “You are also able to reduce neuropathy with the cooling of the hands and feet in patients receiving taxanes, and this has been a welcoming additional benefit in our patients.”
Hand-Foot Skin Reaction
Patients treated with VEGFR or PDGFR inhibitors are susceptible to developing a reaction in the palms and soles that is characterized by painful blisters and hyperkeratosis. Hand-foot syndrome usually happens within the first 6 weeks of treatment and can be so severe that the pain prevents patients from walking.
Once it has occurred, topical anesthetics and anti-inflammatory drugs are used to manage the sores. As prophylactic therapy, the only study with evidence of treatment benefit was a case of 871 patients with advanced hepatocellular cancer treated with sorafenib (Nexavar) who also received best supportive care with or without 10% urea-based cream 3 times daily. All-grade incidence of hand-foot syndrome was lower in the group that received the urea-based cream (56.0%) versus the group that received best supportive care alone (73.6%) at 12 weeks.5
“This is an inexpensive intervention,” Lacoutre said. “Patients would apply this on their palms and soles 3 times per day.”
References
- Lacouture ME. Clinical update: skin related toxicities in cancer care. Data made available as part of the virtual platform for the 2020 Community Oncology Alliance Annual Conference; April 23-24, 2020.
- Lacouture ME, Keefe DM, Sonis S, et al. A phase II study (ARCHER 1042) to evaluate prophylactic treatment of dacomitinib-induced dermatologic and gastrointestinal adverse events in advanced non-small-cell lung cancer. Ann Oncol. 2016;27(9):1712‐1718. doi:10.1093/annonc/ mdw227
- Winther D, Saunte DM, Knap M, Haahr V, Jensen AB. Nail changes due to docetaxel—a neglected side effect and nuisance for the patient. Support Care Cancer. 2007;15(10):1191‐1197. doi:10.1007/s00520-007-0232-0
- Scotté F, Tourani JM, Banu E, et al. Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol. 2005;23(19):4424‐4429. doi:10.1200/ JCO.2005.15.651
- Ren Z, Zhu K, Kang H, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(8):894‐900. doi:10.1200/JCO.2013.52.9651

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.