Investigators Employ a Range of Novel and Established Systemic Therapies for Advanced Glioblastoma
Treatment of glioblastoma has seen few advancements since the approval of temozolomide plus radiotherapy in 2005 for newly diagnosed disease. Recently, promising clinical data have reinvigorated hope for novel therapies in this tumor type.
Roger Stupp, MD

Looking at the long list of different therapeutic modalities being explored across treatment settings, thought leaders in the field eagerly await positive trial results that will determine the next breakthrough.
The complexity of glioblastoma has eluded many traditional treatment approaches through the years. Since 2005, the standard of care for patients with newly diagnosed glioblastoma following surgery has been chemotherapy with temozolomide (Temodar) and radiation therapy.1,2 Novel approaches to glioblastoma treatment are poised to change the paradigm of therapy in a field formerly plagued by limited options.
“No one thought temozolomide or tumor-treating fields were going to be a breakthrough,” said Roger Stupp, MD, in an interview with Targeted Therapies in Oncology, expressing hope that these numerous novel modalities may provide fresh advances in glioblastoma treatment. Stupp is chief of neuro-oncology in the Department of Neurology at Northwestern University Feinberg School of Medicine and co-director of the Northwestern Medicine Lou and Jean Malnati Brain Tumor Institute of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago, Illinois.
Gene and Viral Therapies Show Promise
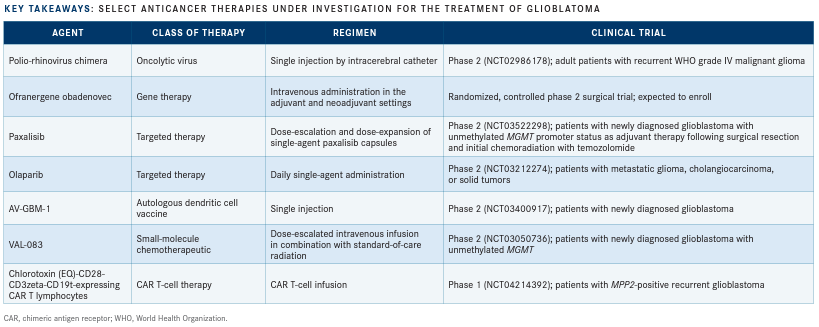
Recombinant nonpathogenic polio-rhinovirus chimera (PVSRIPO) immunotherapy uses a live modified attenuated poliovirus to directly target malignant cells. PVSRIPO specifically targets CD155, a receptor found predominantly on tumor cells, causing cell lysis and activating the immune response toward tumor cells.3
In a paper published in the New England Journal of Medicine, investigators led by Annick Desjardins, MD, evaluated the use of PVSRIPO by convection-enhanced delivery via a catheter inserted directly into the tumor after biopsy in 61 patients with recurrent World Health Organization (WHO) grade IV malignant glioma in a dose-escalation and dose-expansion clinical trial. Results of the phase 1 trial (NCT01491893) were compared with a historical control at the same institution and demonstrated a median overall survival (OS) of 12.5 months (95% CI, 9.9-15.2) in the PVSRIPO group versus the historical control group at 11.3 months (95% CI, 9.8-12.5).4
Notably, the OS rate in the experimental group plateaued between 24 and 36 months, with an OS rate of 21% at both time points; conversely, the OS rate in the historical control group declined over time, at 14% at 24 months and 4% at 36 months.4
Stupp astutely reviewed the survival data. “Looking at the [supplementary] appendix for patients who have been surviving longer than 2 years, all but 1 patient had favorable prognostic tumor characteristics based on either an isocitrate dehydrogenase [IDH] mutation or methylguanine-DNA methyltransferase [MGMT] promoter methylation status. We would expect these patients to live beyond 2 years,” he said.
Stupp noted, “it would be interesting to know for all patients the survival they had from initial diagnosis.”
Grade 1/2 adverse events (AEs) attributed to PVSRIPO occurred in most patients (69%). Common AEs included headache, pyramidal tract syndrome, seizure, dysphasia, and cognitive disturbance. Because the virus is modified, normal tissue is not affected, with no evidence of viral shedding or neuropathogenicity such as encephalomyelitis, poliomyelitis, meningitis, or systemic autoimmune reactions in any patients.4
A phase 2 trial examining the safety and efficacy of PVSRIPO in adult patients with recurrent WHO grade IV malignant glioma is currently recruiting (NCT02986178).
Another agent at the forefront of glioblastoma treatment is ofranergene obadenovec (VB-111), an anticancer gene therapy that induces targeted tumor vascular endothelial cell lysis and leads to an immune response targeted to tumor cells.
VB-111 was studied in patients with recurrent glioblastoma in a phase 1/2 prospective, open-label, dose-escalating, multicenter study (NCT01260506). Patients were stratified into 4 treatment groups: a subtherapeutic group that received initial doses below the therapeutic dose of 1 × 1013 viral particles; a limited exposure (LE) group receiving VB-111 monotherapy every 56 days until progression; a primed combination group receiving VB-111 monotherapy every 56 days until progression, followed by VB-111 plus bevacizumab (Avastin) every 2 weeks; and an unprimed combination group receiving VB-111 every 28 days plus bevacizumab every 2 weeks.5
The primed combination group demonstrated extended survival compared with the LE and unprimed combination groups, with a median OS of 414 days versus 223 days (HR, 0.48; 95% CI, 0.23-0.998; P = .043) and 141.5 days (HR, 0.24; 95% CI, 0.09-0.66; P = .0056), respectively.5
Progression-free survival (PFS) was also prolonged in the primed combination group (90 days) compared with the LE group (60 days; HR, 0.36; 95% CI, 0.140.93; P = .032). VB-111 proved to be safe, with no dose-limiting toxicities, and the maximum tolerated dose was not reached.5
The most common AEs across all groups included pyrexia, fatigue, chills, headache, nausea, and seizure. Additionally, postinfusion febrile reaction was associated with improved survival, with a median OS of 448 days in patients with fever versus 235 days in those without (HR, 0.34; 95% CI, 0.18-0.62; P <.001).5
Based on positive findings in early clinical trials such as the one described above, the pivotal phase 3 GLOBE trial (NCT02511405) evaluated the combination of VB-111 and bevacizumab versus bevacizumab alone in patients with recurrent glioblastoma. The study failed to demonstrate a benefit of the combination versus bevacizumab monotherapy, however, with median OS of 6.8 months versus 7.9 months (HR, 1.20; 95% CI, 0.91-1.59; P = .19) and objective response rates of 27.3% versus 21.9%, respectively (P = .26).6
“Trials in the recurrent setting are difficult to conduct,” Stupp said. “The phase 3 trial was appropriately designed; however, the median survival was on the lower end [for] recurrent disease, demonstrating lack of signal activity.”
The conflicting survival results from the phase 1/2 and phase 3 trials may be explained by the lack of VB-111 priming in the phase 3 trial, with the GLOBE investigators hypothesizing that coadministration of VB-111 and bevacizumab may have blocked VB-111’s antitumor effect.6 However, given the impressive results from previous studies with VB-111, the agent was set to move forward in a 3-arm phase 2 surgical trial examining its use as neoadjuvant and adjuvant treatment for recurrent glioblastoma. The study will evaluate early immunologic pharmacodynamic parameters to examine the primary end point of the effect of neoadjuvant VB-111 on density of tumor- infiltrating T cells.7
Targeted Agents Move Forward
In the area of oral drug therapies for glioblastoma, 2 targeted agents currently under investigation stand out.
Paxalisib (formerly GDC-0084) is a novel targeted agent that potently inhibits the PI3K pathway, which is altered in more than 85% of glioma cases. Its ability to traverse the bloodbrain barrier (BBB) makes this drug a particularly attractive option in glioblastoma.8
A dose-escalation and -expansion phase 2 study in patients with newly diagnosed glioblastoma with unmethylated MGMT promoter status is evaluating paxalisib as adjuvant therapy following surgical resection and chemoradiation with temozolomide (NCT03522298). Interim results from the escalation cohort (n = 9) demonstrated a median OS of 17.7 months compared with 12.7 months for similar patients treated with temozolomide in previous work. Additionally, median PFS in all evaluable patients (n = 30) was 8.5 months with paxalisib versus 5.3 months with temozolomide. Common AEs reported for paxalisib included hyperglycemia, oral mucositis, and low-grade rash.9
“From the press release, the data look encouraging for testing the agent in the up-front setting,” Stupp said. “However, additional results are necessary, including full patient characteristics and how MGMT status was [collected], in order to determine the outcome data.”
In addition to paxalisib, “there has been renewed interest in PARP inhibition in the field of glioblastoma over the last few years,” Stupp said. “Veliparib has the advantage that it crosses the blood–brain barrier better than the other agents. That is one thing that is key, but results to date in unselected populations have not been conclusive.”
The PARP inhibitor olaparib (Lynparza) has shown efficacy in glioma cells with IDH mutations and has enhanced the effects of temozolomide when used in combination.10 A phase 2 study (NCT03212274) is evaluating the use of olaparib in the metastatic or treatment-refractory disease setting. Eligible patients may have several solid tumor types, including glioma, with IDH1/2 mutations.
Immunotherapy, Chemotherapy, and Vaccines
The era of creative innovation in glioblastoma therapy has also ushered in multiple immunotherapeutic agents and vaccines in clinical trials that directly target malignant cells while minimizing negative effects on normal tissue.
AV-GBM-1 is a novel immunotherapy that uses autologous dendritic cells loaded with autologous tumor-associated antigens, allowing it to directly target stem cells responsible for tumor growth. A single-arm, phase 2 clinical trial included patients with newly diagnosed disease who had recovered from surgery and were about to start concurrent chemotherapy and radiation (NCT03400917). Interim results demonstrated improved survival in the AV-GBM-1 group (n = 50) compared with standard of care (n = 287), with an OS rate of 76% at both 12 months and 15 months compared with 61% at 12 months and 48% at 15 months, respectively.11 When trial data were initially presented in November 2019, the first 16 patients demonstrated an immune response associated with a decrease in tumor biomarkers.12
An open-label, single-arm, phase 2 clinical trial (NCT03050736) is evaluating the maximum tolerated dose of VAL-083, a small- molecule chemotherapeutic, in combination with the standard-of-care radiation regimen used to treat patients with newly diagnosed glioblastoma with unmethylated MGMT promoter status. According to an interim analysis, of the 15 patients assessed for tumor progression, 7 demonstrated complete response and 8 had stable disease. The most common AE was myelosuppression, which was consistent with the investigators’ previous experience. VAL-083 functions as a bifunctional DNA-targeting agent that induces double-strand breaks and circumvents MGMT-mediated repair.13
Another innovative approach being examined for treatment of glioblastoma is chimeric antigen receptor (CAR) T-cell therapy, with one such agent currently under evaluation in a phase 1 dose-escalation trial of patients with recurrent or progressive glioblastoma following standard therapy (NCT04214392). The therapy in this trial has an unusual CAR design with a recognition domain derived from chlorotoxin, a component of scorpion venom. The trial’s primary goals are to evaluate the safety and efficacy of the therapy and any dose-limiting toxicities. In cell-based assays and animal models, the chlorotoxin-directed CAR T cells recognized and eliminated glioblastoma cells without toxic effects on nontumor cells and other organs.14
Investigators Uncover Potential Prognostic Biomarkers
In addition to efforts to develop multiple novel agents for treating glioblastoma, clinicians have worked toward implementing liquid biopsy as a minimally invasive tool for molecular profiling and disease monitoring.
In a single-center, post hoc analysis of 42 patients with newly diagnosed glioblastoma in a previously enrolled prospective cohort, MRI scans were used in conjunction with histopathology to assess plasma cell-free DNA (cfDNA) concentration and circulating tumor DNA (ctDNA) detection. The investigators were seeking to identify factors associated with cfDNA concentration, which is typically low in glioblastoma and can limit the ability to detect ctDNA.15
“In addition to anatomical imaging, we looked into other advanced imaging metrics, including dynamic contrast-enhanced MRI, which measures the blood–brain barrier permeability, and we found a significant correlation between the volume of the tumor [and] increased permeability of blood–brain barrier beyond a certain threshold compared with contralateral white matter and cfDNA,” Seyed Ali Nabavizadeh, MD, lead study author as well as assistant professor of radiology at the Hospital of the University of Pennsylvania, in Philadelphia, said in an interview with Targeted Therapies in Oncology.
Results of the study demonstrated that factors associated with increased plasma cfDNA concentration included BBB disruption (P = .001), increased tumor macrophage density (P = .011), and increased tumor vessel size (P = .012).15
“We also looked at mutations in ctDNA, and we found that there was an inverse relationship between the perivascular macrophages and the detection of ctDNA in the plasma,” Nabavizadeh said. “atients who had higher perivascular macrophage density had lower ctDNA detection in the plasma, which indicates that the immune microenvironment of the tumor, mainly tumor associated macrophages, can affect the detectability of ctDNA.”
The identification of plasma cfDNA- associated characteristics in this study may help clinicians select patients who will benefit from liquid biopsy, a minimally invasive approach used in conjunction with imaging studies to assess disease progression. Nabavizadeh noted that “liquid biopsy could play a more vital role in the future of glioblastoma if advances in targeted treatment provided improved survival outcomes [for patients who may] be medically managed with long-term follow-up. In addition, if future studies corroborate our findings that liquid biopsy can inform us about tumor-associated macrophages, that could be an additional application of liquid biopsy for monitoring targeted treatment against tumor-associated macrophages.
“More advanced imaging, such as amino acid PET and immuno-PET imaging, can provide the opportunity for real-time monitoring of the tumor-immune microenvironment and metabolism and would be important to be included in future studies,” Nabavizadeh added.
Another imaging study was able to identify biomarkers predicting PFS to chemotherapy response in patients with glioblastoma. Retrospective MRI data from newly diagnosed patients were analyzed and a radiomic risk score (RRS) was built using 25 radiomic features from the tumor habitat captured on routine pretreatment MRI. It was trained on data from 130 patients and tested with data from 73.
The RRS was associated with PFS in both the training cohort (P <.00001) and the independent test group (P = .0117).16 Additionally, augmenting the MRI-based RRS with clinical parameters such as age and gender, molecular features such as MGMT and IDH status, and extent of resection improved the prediction of PFS in training (P <.0001) and test sets (P = .03).
This was also the first clinical study to bridge the gap between tumor radiomic features and genetics. Using gene ontology and single-sample gene set enrichment analyses, significant correlations were found between the radiomic features and molecular signaling pathways associated with cell differentiation, cell adhesion, and angiogenesis (P <.05).16 The results of this study may provide further insight into ways to predict tumor recurrence and evaluate the efficacy of chemoradiation therapy in patients with glioblastoma.
“We’re still in the exploratory phase of advancing glioblastoma treatment, and what we need are definitive results [from randomized clinical trials],” Stupp said. Meanwhile, clinicians and patients eagerly await new treatment approaches that will extend survival.
References
- NCCN. Clinical Practice Guidelines in Oncology. Central nervous system cancers, version 2.2020. Accessed May 13, 2020. https://bit.ly/3bo6Rfm
- Temodar approved for treating GBM in combination with radiotherapy. Cancernetwork. Published April 1, 2005. Accessed May 3, 2020. https://bit.ly/2Z10dJh
- University Hospitals Seidman Cancer Center treats its first glioblastoma patient with genetically modified poliovirus. News release. University Hospitals; January 24, 2020. Accessed April 26, 2020. https://bit.ly/3bpKGpf
- Desjardins A, Gromeier M, Herndon JE 2nd, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150-161. doi:10.1056/NEJMoa1716435
- Brenner AJ, Peters KB, Vredenburgh J, et al. Safety and efficacy of VB-111, an anticancer gene therapy, in patients with recurrent glioblastoma: results of a phase I/II study. Neuro Oncol. 2020;22(5):694-704. doi:10.1093/neuonc/noz231
- Cloughesy TF, Brenner A, de Groot JF, et al; GLOBE Study Investigators; Patrick YW. A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro Oncol. 2020;22(5):705-717. doi:10.1093/neuonc/noz232
- Cloughesy T, Butowski N, Harats D, et al. Clinical trial in progress: a study of neo-adjuvant and adjuvant VB-111 for treatment of recurrent GBM. Neuro Oncol. 2019;21(suppl 6;abstr ATIM-09). doi:10.1093/neuonc/noz175.009
- Paxalisib. Kazia Therapeutics. Accessed April 26, 2020. https://bit.ly/2Wr7v7y
- Kazia’s paxalisib shows positive overall survival signal in phase II glioblastoma study. News release. Kazia Therapeutics Limited; April 7, 2020. Accessed April 26, 2020. https://prn.to/35TZkE5
- PARP inhibitors may be effective in brain, other cancers with IDH mutations. NIH National Cancer Institute. Published April 24, 2017. Accessed April 26, 2020. https://bit.ly/2WsqH4T
- AIVITA Biomedical provides update of ongoing phase 2 glioblastoma trial from year-end survival analysis. News release. AIVITA Biomedical; April 8, 2020. Accessed April 29, 2020. https://bit.ly/2Aqu1oH
- AIVITA Biomedical announces new positive phase 2 glioblastoma data at SITC Annual Meeting podium presentation. News release. AIVITA Biomedical; November 12, 2019. Accessed April 29, 2020. https://bit.ly/3cuY7Wa
- Chen Z-p, Guo C, Yang Q-y, et al. Clinical trial of VAL-083 in newly diagnosed MGMT-unmethylated GBM: half-way report. Neuro Oncol. 2019;21(suppl_6;abstr ACTR-06). doi:10.1093/neuonc/noz175.050
- Marquez L. From scorpion to immunotherapy: City of Hope scientists repurpose nature’s toxin for first-of-its kind CAR T cell therapy to treat brain tumors. News release. City of Hope; March 4, 2020. Accessed April 29, 2020. https://bit.ly/2WQQx1w
- Nabavizadeh SA, Ware JB, Guiry S, et al. Imaging and histopathologic correlates of plasma cell-free DNA concentration and circulating tumor DNA in adult patients with newly diagnosed glioblastoma. Neurooncol Adv. 2020;2(1):vdaa016. doi:10.1093/noajnl/vdaa016
- Beig N, Bera K, Prasanna P, et al. Radiogenomic-based survival risk stratification of tumor habitat on Gd-T1w MRI is associated with biological processes in glioblastoma. Clin Cancer Res. 2020;26(8):1866-1876. doi:10.1158/1078-0432.CCR-19-2556
