Comorbid Toxicities Can Cloud Differential Diagnosis for Immune-Related AEs
A review of selected trials looking at frequencies of grade 3 irAEs suggests that renal and bone marrow toxicities occur more frequently with the use of PD-1 and PD-L1 inhibitors compared with agents blocking CTLA-4
Lillian L. Siu, MD

As the use of combined immunotherapies evolves into a mainstay of treatment across cancer settings, the agents’ unique immune-related adverse effects (irAEs) pose a challenge to management because of other patient comorbidities. This is particularly problematic for health careteams in emergency departments, who need to quickly assess and differentiate between irAEs and toxicities associated with comorbidities.
Lillian L. Siu, MD, a clinician scientist in the Cancer Clinical Research Unit at the University Health Network Princess Margaret Cancer Centre in Toronto, Canada, provided a framework for distinguishing between the 2 sets during a presentation for the National Institutes of Health–American Association of Cancer Research Cancer, Autoimmunity, and Immunology Conference, which was held virtually.1
“Patients who come to the clinic often have comorbidities in addition to their cancer,” Siu said. “They’re often receiving other drugs at the same time, so to differentiate [an adverse] effect that is related to their immunotherapy versus all the other conditions can be quite challenging.”
Almost every organ is a target for irAEs.2 Although dermatologic irAEs often present as rash, vitiligo pruritis, and other observable symptoms, Siu noted that other signs are not as clinically obvious. In particular, central nervous system irAEs can be subtle.
“For example, patients may be suffering with hypophysitis, but they may not present with obvious symptoms that are easily recognizable unless the clinician has a high index of suspicion,” Siu said.
Siu’s protocol starts with educating the patient to promptly inform the treating physician and team of new signs and symptoms. Patients may not be forthcoming about these AEs because they think their treatment may be halted as a result, so offering reassurance that symptom relief can help them continue treatment is important.
Frontline health care teams need to be aware of irAEs during the differential diagnosis. Consulting relevant specialists such as endocrinologists and rheumatologists can be helpful. The differential diagnosis should include disease-related causes, intercurrent illnesses, drug-related causes associated with immunotherapies and nonimmunotherapies, and other concomitant medications.
Additional ways to differentiate irAEs from other AEs include patient factors, such as underlying history that raises risks of conditions such as connective tissue disorders, vasculitis, and severe autoimmune disorders; a genetic predisposition; the microbiome environment; and cancer type.
A review of selected trials looking at frequencies of grade 3 irAEs suggests that renal and bone marrow toxicities occur more frequently with the use of PD-1 and PD-L1 inhibitors compared with agents blocking CTLA-4 (TABLE).2 Gastrointestinal toxicities are present with both PD-1/PD-L1 and CTLA-4 inhibitors; when these agents are combined, toxicities increase.
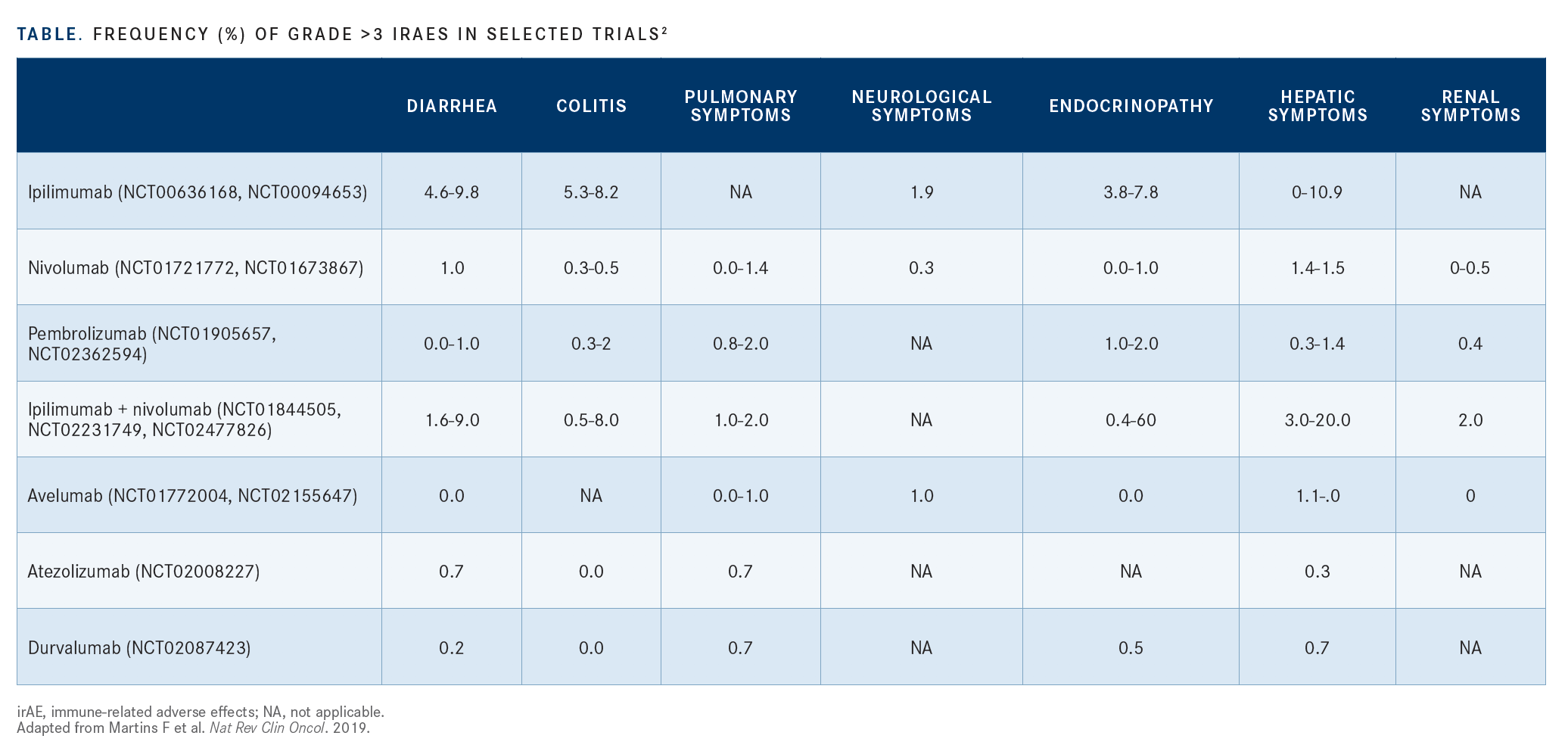
“These toxicities are not exclusive to PD-1/PD-L1 or CTLA-4 agents,” Siu said. “We’re trying to understand the pattern of these toxicities.”
Certain cancers are more likely to result in irAEs than others, according to Siu.3
“Patients with lung cancer are more likely to experience gastrointestinal and skin toxicities, whereas patients with melanoma are more likely to develop pneumonitis,” she said. In renal cell carcinoma, patients are more likely to demonstrate skin, gastrointestinal, and arthralgia toxicities compared with patients with lung cancer or melanoma.
A review of phase 1 trials at Princess Margaret Cancer Centre that involved immunotherapy agents revealed that dose-limiting toxicities typically occur during the first cycle of treatment.4 Siu said that the highest risk of first-onset clinically significant AEs (csAEs) was during the first 4 weeks of treatment compared with the period from 4 weeks to the end of treatment (odds ratio, 3.13; 95% CI, 1.95-5.02). The median time to first onset of csAEs was significantly shorter in patients who received combination immunotherapy than in patients receiving monotherapy (32 vs 146 days; P < .001).
“Physicians need to be aware that these clinically significant [AEs] may not occur in the first 4 weeks of therapy, and if you don’t see them in that time period, it doesn’t mean the patient is fine,” Siu said. “We need to always be on hyperalert, especially when treating patients with monotherapy, because AEs can take some time to appear.”
Siu concluded by recommending the consideration of broad differential diagnosis and maintain a high index of suspicion when patients present with toxicities. She also emphasized the importance of recognizing patterns with certain irAEs, which tend to present with certain classes of drugs. In addition, she said, reviewing the literature that includes AE reporting in clinical trials involving immunotherapy-based combinations suggests that toxicity appears to be additive when certain drugs are combined.
References:
1. Siu LL. Differentiating between irAEs and other AEs in combination therapies. Data made available as part of the virtually held National Institutes of Health–American Association of Cancer Research Cancer, Autoimmunity, and Immunology Conference; March 23-24, 2020.
2. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563‐580. doi:10.1038/s41571-019-0218-0
3. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377‐2385. doi:10.1093/annonc/mdx286
4. Kanjanapan Y, Day D, Butler MO, et al. Delayed immune-related adverse events in assessment for dose-limiting toxicity in early phase immunotherapy trials. Eur J Cancer. 2019;107:17. doi:10.1016/j.ejca.2018.10.017

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.