Evaluating the TKI Inhibitors in Fusion-Driven NSCLC
In a presentation during the 41st Annual Chemotherapy Foundation Symposium Innovative Cancer Therapy for Tomorrow conference, Jessica Lin, MD, discussed the landscape of targeted therapies in driver mutations in non–small cell lung cancer.

For some non–small cell lung cancers (NSCLC), oncogenic driver mutations play a major part in carcinogenesis and tumor progression, particularly in adenocarcinomas. The greater understanding of the role of ALK, ROS1, RET, and NTRK fusions and subsequent development of tyrosine kinase inhibitors (TKIs) have revolutionized the treatment landscape for patients with NSCLC who harbor these particular mutations.
In a presentation during the 41st Annual Chemotherapy Foundation Symposium Innovative Cancer Therapy for Tomorrow conference, Jessica Lin, MD, assistant professor of medicine, Harvard Medical School, and attending physician, Center for Thoracic Cancers, Massachusetts General Hospital, both in Boston, discussed the landscape of targeted therapies in driver mutations in NSCLC.1
ALK Fusion-Positive Lung Cancer
The first-generation TKI ALK inhibitor, crizotinib (Xalkori), was approved in 2011 for the treatment of patients with ALK-positive locally advanced or metastatic NSCLC as detected by a companion diagnostic.2 This was followed by second- generation TKIs ceritinib (Zykadia), alectinib (Alecensa), and brigatinib (Alunbrig), and the current, third-generation TKI lorlatinib (Lorbrena).
“Each successive generation has increased potency against ALK, increased central nervous system [CNS] penetration and activity, and broader coverage of ALK resistance mechanisms,” said Lin, who is also an attending physician at the Henri and Belinda Termeer Center for Targeted Therapies at Massachusetts General Hospital.
Second-generation ALK inhibitors such as alectinib and brigatinib have supplanted crizotinib as the preferred first-line agent. Third-generation lorlatinib has gained first-line privileges as well. “What do we do now? Which one do we choose?” Lin asked. Turning to key efficacy data may help inform treatment decisions, she added.
Jessica Lin, MD
Assistant Professor of Medicine Harvard Medical School Attending Physician Center for Thoracic Cancers Massachusetts General Hospital Attending Physician Henri and Belinda Termeer Center for Targeted Therapies Massachusetts General Hospital Boston, MA

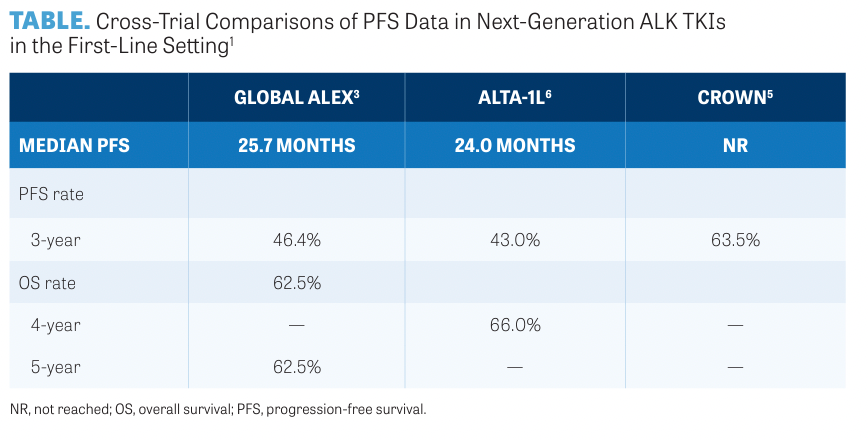
The phase 3 ALEX trial (NCT02075840) compared alectinib with crizotinib and demonstrated that after a median follow-up of 17.6 months (for crizotinib) and 18.6 months (for alectinib), disease progression or death occurred in 62 of 152 patients (41%) in the alectinib group compared with 102 of 151 patients (68%) in the crizotinib group.3 Median progression- free survival (PFS) was 10.9 months for crizotinib (95% CI, 0.3-49.8) and 34.8 months for alectinib (95% CI, 0.5-50.7). Investigators reported an HR of 0.43 (95% CI, 0.32-0.58). In patients with baseline CNS metastases, the median PFS was 25.4 months (95% CI, 9.2-not evaluable) for alectinib vs 7.4 months (95% CI, 6.6-9.6) for crizotinib (HR, 0.37; 95% CI, 0.23-0.58).
The phase 3 CROWN trial (NCT03052608),4 which compared lorlatinib with crizotinib in the first-line setting, demonstrated lorlatinib's PFS superiority over crizotinib. The interim analysis showed that the median PFS for lorlatinib was not yet reached vs 9.3 months for crizotinib (HR, 0.28; 95% CI, 0.19-0.41).5
In the phase 3 ALTA-1L trial (NCT02737501), 275 patients were randomly assigned to receive either brigatinib (n = 137) or crizotinib (n = 138).6 Median PFS was 24.0 months in patients treated with brigatinib and 11.2 months in patients treated with crizotinib (HR, 0.48; 95% CI, 0.35-0.66; P < .0001). Comparisons of PFS data for next- generation ALK TKIs in the frontline setting are summarized in the TABLE.1
CNS Efficacy
“Another important aspect to consider is the level of CNS efficacy,” Lin said. Most next-generation ALK inhibitors have good CNS efficacy. In particular, lorlatinib has been shown to achieve the highest level of CNS penetration among the approved ALK inhibitors.
“This does translate to higher levels of intercranial complete response [CR] rates and the ability to delay or prevent brain metastases,” Lin said. “In the CROWN study, investigators evaluated the cumulative incidence of brain metastases. Among the 112 patients who did not have brain metastasis at baseline, only 1 patient developed CNS involvement on first-line lorlatinib, which is remarkable for that patient population,” Lin continued.5
ROS1 TKIs
There are 2 approved agents for patients with ROS1 fusion-positive lung cancer: crizotinib and entrectinib (Rozlytrek). Both agents have demonstrated similar response rates.
In the phase 1 PROFILE 1001 study (NCT00585195) that evaluated crizotinib, the objective response rate (ORR) was 72% (95% CI, 58%-83%), including 6 confirmed CRs and 32 confirmed partial responses; 10 patients had stable disease.7 Responses were durable with a median duration of response of 24.7 months (95% CI, 15.2-45.3).
Entrectinib demonstrated an ORR of 68% (95% CI, 60.2%-74.8%) and a median duration of response of 20.5 months, according to Alexander Drilon, MD, and colleagues.8 In this combined analysis study, the median PFS was 15.7 months and the median OS was 47.8 months.
“Both these agents are very good for this disease but there are distinctions, including CNS efficacy,” Lin said. Crizotinib is not as active as entrectinib, which has an intracranial response rate of 80% in patients with baseline measurable CNS metastases. Also, median intracranial PFS is 8.4 months (range, 6.4-13.8).8
Regarding adverse events (AEs), vision disorders, nausea, edema, diarrhea, and vomiting characterize crizotinib, but these tend to occur early in treatment and are transient. For entrectinib, common AEs include weight increase, paresthesia, myalgia, and blood creatinine increase.
RET Fusion-Positive Lung Cancer
The current standard of initial therapy for these patients is selpercatinib (Retevmo) and pralsetinib (Gavreto). In platinum- pretreated patients, selpercatinib had an ORR of 61% (95% CI, 55%-67%)9 and pralsetinib had an ORR of 59% (95% CI, 50%-67%).10 In treatment-naive patients, selpercatinib had an ORR of 84% (95% CI, 73%-92%) and pralsetinib had an ORR of 72% (95% CI, 60%-82%).9,10
At the recent European Society for Medical Oncology Congress 2023, Herbert Ho Fung Loong, MD, and colleagues presented findings from a phase 3 study (NCT04194944) of first-line selpercatinib vs chemotherapy and pembrolizumab (Keytruda) in RET fusion-positive NSCLC.11 Selpercatinib achieved a statistically significant and clinically meaningful improvement in PFS compared with chemotherapy plus pembrolizumab. “These findings cemented in my mind that we should be using selpercatinib and pralsetinib, RET-selective inhibitors, rather than chemo-immunology approaches in patients with RET fusion-positive lung cancer,” Lin said.
NTRK Fusion-Positive Lung Cancer
The agents larotrectinib (Vitrakvi) and entrectinib deliver robust efficacy and prolonged responses in this patient population.12,13 ORR for larotrectinib was 74% (95% CI, 54%-90%), according to findings from Lin JJ et al12 and 64.5% (95% CI, 45.4%-80.8%) for entrectinib, according to Cho BC et al.13
As she concluded her presentation, Lin noted that there are highly effective, FDA-approved targeted therapies available in the first line for metastatic NSCLC harboring ALK, ROS1, RET, and NTRK gene fusions. With a number of agents to consider, biomarker testing with next-generation sequencing at initial diagnosis becomes even more important. The role of TKIs in earlier-stage, fusion-driven lung cancers is undergoing evaluation.
REFERENCES:
1. Lin J. Targeted therapy for fusion-driven (ALK/RET/ROS1/NTRK) lung cancer. Presented at: 41st Annual CFS Chemotherapy Foundation Symposium Innovative Cancer Therapy for Tomorrow conference; November 8-10, 2023; New York, NY.
2. Kazandjian D, Blumenthal GM, Chen HY, et al. FDA approval summary: crizotinib for the treatment of metastatic non–small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19(10):e5-e11. doi:10.1634/theoncologist.2014-0241
3. Peters S, Camidge DR, Shaw AT, et al; ALEX Trial Investigators. Alectinib versus crizotinib in untreated ALK-positive non–small cell lung cancer. N Engl J Med. 2017;377(9):829-838. doi:10.1056/NEJMoa1704795
4. Shaw AT, Bauer TM, de Marinis F, et al; CROWN Trial Investigators. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018-2029. doi:10.1056/NEJMoa2027187
5. Solomon B, Bauer TM, De Marinis F, et al. Lorlatinib vs crizotinib in the first-line treatment of patients (pts) with advanced ALK-positive non–small cell lung cancer (NSCLC): results of the phase III CROWN study. Ann Oncol. 2020;31(suppl 4):S1180-S1181. doi:10.1016/j.annonc.2020.08.2282
6. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: final results of phase 3 ALTA-1L trial. J Thorac Oncol. 2021;16(12):2091-2108. doi:10.1016/j.jtho.2021.07.035
7. Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non–small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121-1126. doi:10.1093/annonc/mdz131
8. Drilon A, Chiu CH, Fan Y, et al. Long-term efficacy and safety of entrectinib in ROS1 fusion-positive NSCLC. JTO Clin Res Rep. 2022;3(6):100332. doi:10.1016/j.jtocrr.2022.100332
9. Drilon A, Subbiah V, Gautschi O, et al. Selpercatinib in patients with RET fusion-positive non–small cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol. 2023;41(2):385-394. doi:10.1200/JCO.22.00393
10. Griesinger F, Curigliano G, Thomas M, et al. Safety and efficacy of pralsetinib in RET fusion-positive non–small cell lung cancer including as first-line therapy: update from the ARROW trial. Ann Oncol. 2022;33(11):1168-1178. doi:10.1016/j.annonc.2022.08.002
11. Loong HHF, Goto K, Solomon BJ, et al. Randomized phase 3 study of first-line selpercatinib versus chemotherapy and pembrolizumab in RET fusion-positive NSCLC. Ann Oncol. 2023;34(suppl 2): S1303. doi:10.1016/j.annonc.2023.10.059
12. Lin JJ, Kummar S, Tan DSW, et al. Long-term efficacy and safety of larotrectinib in patients with TRK fusion-positive lung cancer. J Clin Oncol. 2021;39(15):9109. doi:10.1200/JCO.2021.39.15_suppl.9109
13. Cho BC, Chiu CH, Massarelli E, et al. Updated efficacy and safety of entrectinib in patients (pts) with locally advanced/metastatic NTRK fusion-positive (fp) non–small cell lung cancer (NSCLC). J Clin Oncol. 2023;41(suppl 16):9047. doi:10.1200/JCO.2023.41.16_suppl.9047
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More