Community Case Forum: Exploring Adjuvant Immunotherapy for RCC
During a Targeted Oncology™ Community Case Forum™ event, Hans Hammers, MD, discussed trials that shed light on the role of adjuvant therapies for patients with stage II and III renal cell carcinoma.
Hans Hammers, MD, PhD
Professor, Department of Internal Medicine
Division of Hematology and Oncology
UT Southwestern Medical Center
Dallas, TX

CASE SUMMARY
A 48-year-old man visited his primary care physician due to persistent fatigue, nausea, and vomiting that had developed after “a cold,” recent weight loss of 15 to 20 lb, and onset of hematuria. A noncontrast CT scan of the abdomen and pelvis showed a large (11.3 cm) right renal mass with extension into the renal vein. CT scan of the chest was negative.
The patient’s medical history included obesity, obstructive sleep apnea, and appendectomy. A former smoker whose mother and sister had lung cancer, he had lost a total of 40 lb during the past year.
Laboratory results
- Hemoglobin: 10.8 g/dL
- Absolute neutrophil count: 7.4 × 103 cells/μL
- Platelet count: 316,000/μL
- Calcium: 9.8 mg/dL
- ECOG performance status: 1
The patient underwent nephrectomy and limited lymph node dissection. His pathology was clear cell renal cell carcinoma (RCC) with renal vein invasion and negative margins. The tumor was grade 4 without sarcomatoid features, staging pT3a pN0 M0.
Targeted Oncology: What are the NCCN (National Comprehensive Cancer Network) guidelines for adjuvant treatment of RCC?
HAMMERS: The populations…[in the guidelines] include patients with stage II disease with high-grade tumors [with or without] sarcomatoid features.1 But the [majority] is stage III disease, and then you can choose between roughly 30% chance of recurrence vs 50% chance of recurrence [without adjuvant therapy].1 That also means that you will treat 50% to 70% of patients who never needed immunotherapy, so just a reminder that there are pros and cons…[to] adjuvant therapy. The debate [surrounding it] is also fueled by several trials that were negative.
Would you review the trials of adjuvant targeted therapy in RCC?
All the trials were negative, except one [S-TRAC (NCT00375674)]. By independent review, it was a positive trial [disease-free survival (DFS) favored sunitinib (Sutent); HR, 0.76; 95% CI, 0.59-0.98; P = .03].2 But by investigator review, it was a negative trial [DFS HR, 0.81; 95% CI, 0.64-1.02; P = .08]. That led to the approval of sunitinib. I typically try to talk patients out of it. I was never a firm believer in sunitinib. It typically delayed recurrence by a year [but] never showed an overall survival [OS] benefit, and patients [disliked] it.
Can you tell us about the trials of adjuvant immunotherapy in RCC?
This is the data set that makes for a good debate…[in] the adjuvant space. In principle, we’re always a little bit more excited about immunotherapy than targeted therapy in the adjuvant space [because of] the sunitinib experience.
KEYNOTE-564 [NCT03142334] has a very strong data set, and it remains unclear why some of these other trials have failed to show DFS benefit in that space.3 The IMmotion010 trial [NCT03024996] was with atezolizumab [Tecentriq].4 I would say the general feeling, at least in RCC, is that PD-1 inhibitors are probably more active than PD-L1 inhibitors, so maybe that played into this…patient selection. DFS was the primary end point.
This is the least understood [trial], to be honest. I personally have felt that nivolumab [Opdivo] plus ipilimumab [Yervoy] would be too toxic in the adjuvant space, but I would never have believed that the [Kaplan- Meier DFS] curve would be negative [for adjuvant atezolizumab].
PROSPER RCC [NCT03055013] was a neoadjuvant treatment with nivolumab.5 There were some patients with non-clear cell histology…and also lower-risk patients, so that could be a good reason why it was negative.
For CheckMate 914 [NCT03138512], one of the arms was reported.6 The nivolumab monotherapy arm is still pending. Nobody understands why, with fairly high-risk patients, nivolumab/ipilimumab was negative in that space. We don’t have a good explanation for that, so that leads to continued discussions about the benefit. The primary end point was DFS, and we want to go into that. The secondary end point was OS, which has not yet been statistically significant.
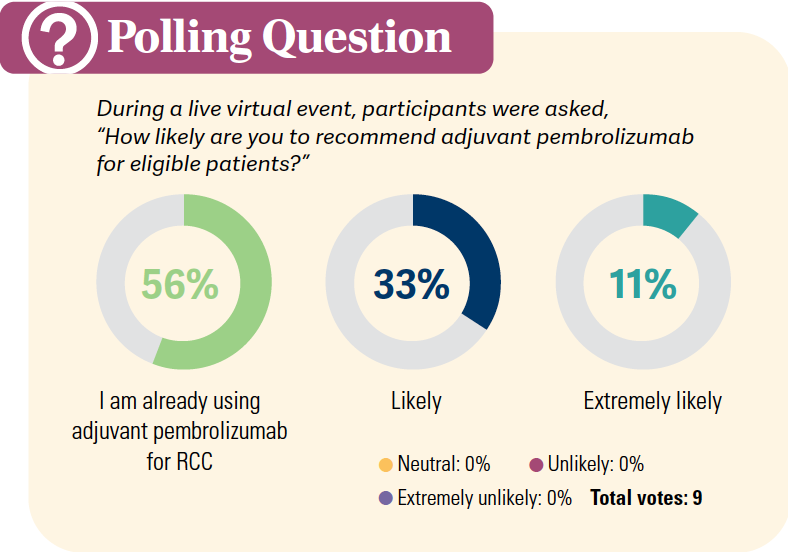
Please discuss KEYNOTE-564 in more detail.
In KEYNOTE-564, patients were given pembrolizumab [Keytruda] 200 mg every 3 weeks for 1 year.3 There were patients with higher-risk disease. The vast majority had stage III disease. It has a curious population that’s probably worth discussing. There were also patients with metastatic disease who were resected, with NED [no evidence of disease], who were enrolled in this trial.
The primary end point was DFS and the key secondary end point was OS. Intermediate-high risk would have been stage II disease with grade 4 RCC or sarcomatoid, or stage III with any grade. Then there was a high-risk population, so that’s stage IV disease, contiguous invasion of other organs, or lymph node-positive disease. The highest-risk group was M1 NED. Curiously, these were patients who had recurrent disease within 1 year of diagnosis, were resected, and then were offered pembrolizumab. In my book, that is a patient population that should not exist. Because in my book, it’s intermediate-risk disease of recurrence. These patients don’t do well without immunotherapy. I would not resect them. I would just treat them with immunotherapy. But that’s my approach.
DFS is clearly much worse for patients with metastatic disease and these higher-risk patients. Most of us would probably think about these patients, [or] at least discuss with them. Then depending on what you believe, you would offer pembrolizumab or not, or offer observation, and may also differentiate on the patient substrate, age, location, follow-up, [adherence], those kinds of things.
[Looking at] patient characteristics…almost 90% of patients were in the stage III disease group. Lymph node-positive disease was single digits. Same with M1 NED, 6%, although quite frankly, a large number of these patients progressed and made up a good proportion of the events.
For geographic location, one-third of patients were in the rest of the world [vs North America and the European Union]. Patients in the rest of the world…in the placebo arm probably would never get immunotherapy if they progressed. This trial did not guarantee access to immunotherapy if you progressed. That could be one of the weaknesses if the OS benefit were weak because it would essentially be a trial of 100% of patients [in the experimental arm] vs probably 70% of patients [in the placebo arm] getting immunotherapy.
In Europe, most patients will get it. In North America most patients will get it. But for the rest of the world, there is a question mark if they will ever get access to immunotherapy. [In terms of] the primary end point of DFS, these are very good data [Table7]. This has been updated now with 30 months’ follow-up.7
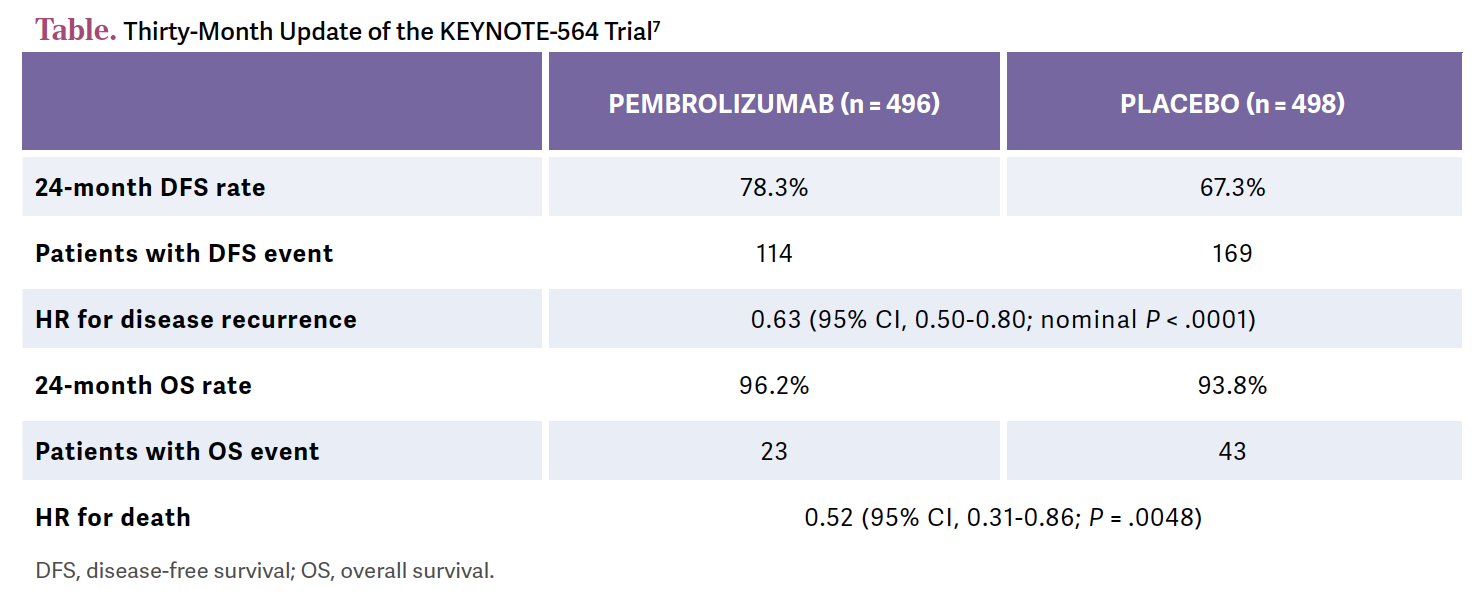
The HR is great, 0.63 [95% CI, 0.50-0.80; nominal P < .0001]. It’s a significant relative risk reduction for the patient. The 24-month DFS rate is a bit more than 10% better. This is a good separation of the [Kaplan-Meier] curve and good maintenance of the curve.
If you look at the subgroups, I don’t think anything stands out, per se, about how we should choose these patients.3,7 PD-L1 status doesn’t [seem to] matter. For tumor grade, there’s a trend in the higher grade that you may benefit, but don’t overread into this because otherwise you would say [the CI] crosses the line and North American patients don’t benefit, but European patients do. It [would make] no sense. Don’t read too much into subgroups; I think across the line there is a benefit.
They broke down the benefit with regard to the HR. The HR improves, the higher the risk. Clearly, if you have M1 NED, the HR is 0.28 [95% CI, 0.12-0.66].8 So the relative risk reduction of recurrence is more than 70% if you give pembrolizumab. To me, this is not proof that adjuvant immunotherapy is the best way. To me, it proves…that you shouldn’t have resected the patients. These patients had recurrence within 1 year. Just treat them with immunotherapy.
I see more and more surgeons who are now excited about this and say, “Well, if you recurred, let me cut out that lung nodule.” If you recurred 6 months after nephrectomy, it’s still systemic disease. You still need immunotherapy. So my personal practice is to spare the patient surgery and just put them on immunotherapy because they need immunotherapy.
With just a single agent, are you undertreating M1 patients?
That’s what I fear. To be honest, if…I had a patient with RCC and…they recurred with 1 or 2 lung nodules—this would be the patient for whom I would think surgical management would be feasible. You don’t have 30 [nodules]; you’re taking out 1 or 2, so it’s oligometastatic. But it’s within 1 year, so it’s not [truly] oligometastatic; it’s just that you see 1 or 2 [nodules]. But these patients are very high risk, so why give them just pembrolizumab if you could give 2 agents that are probably more effective? In fact, you could consider nivolumab and ipilimumab. That might be more durable than PD-1 inhibition alone.
A lot of my colleagues at The University of Texas Southwestern [Medical Center] and in other places share the sense that this is a patient population…[in] whom we should spare the surgery and just treat with systemic therapy. It makes no sense to cut out tumors that recur within 1 year.
It’s a different story if you’re 2 or more years out. That is probably true oligometastatic disease. You have a different biology, recurrence, and tempo, and then stereotactic radiation can play a role, and surgery can play a role. You can then discuss if you want to use that.
The dataset was not achieved, but some other trials are looking at that In fact, some of the trials were negative. A lot of metastatic patients were outside of the 1-year window, so 2 or 3 years resected. Again, those trials actually were negative. Now, the question is, how much does [high-risk status] drive some of the benefit in these studies? Since it’s a subset analysis, so the benefit as outlined here is maintained. If you exclude [metastatic patients] and just look at M0 with high risk or M0 with intermediate risk, clearly there is still a benefit of 30% or more in reduction of relative risk, independent of the M1 population.8 But this is something that is discussed. I would not send them to a surgeon; I would just stop there.
How does adjuvant immunotherapy influence OS in patients with RCC?
OS [leads to] another debate.3,7 If you treat breast cancer in the adjuvant setting, if you treat colon cancer in the adjuvant setting, or if you treat bladder cancer in the neoadjuvant setting, you typically talk about OS benefit.
This is still one of the questions: What are they going to do if there’s no median OS benefit? If you treat patients early in the adjuvant space, they may live as long as if you had waited until the disease came back and treated them. There might not be a difference.
[Although] it can be useful to prevent recurrence and the [effects] of the metastatic recurrence itself in the spine, etc, it’s not yet clear that patients are actually going to live longer by…[receiving] immunotherapy super early. The reason…is probably because it’s a completely different mechanism of action. In breast cancer, it’s very clear.
If you give cytotoxic chemotherapy in the adjuvant space, you will cure patients. If you give it later, when you can see gross metastatic disease, the same chemotherapy doesn’t cure anyone. It’s different for immunotherapy. We [can] cure patients with gross metastatic disease.
This is one of the ongoing debates in the field: How can we do better with patient selection? One of the ways…is to identify patients with minimal residual disease. If you look at the bladder cancer data, IMvigor010 [NCT02450331] for example, you could see that with circulating tumor DNA, you could identify the patients who benefited from immunotherapy.9 My hope is that [the preference for] treating everyone will go away, and we will focus more on discussions of minimal residual disease, identifying residual cancer, for example. That’s the big picture.
These are discussions that [physicians] are having, [saying,] “Well, you’re not going to live any longer, necessarily. At least we don’t have the data yet. I can delay your recurrence, but it’s not clear you’re actually going to live longer if I give you treatment now.” It may change, but those are the data so far.
What are the safety and tolerability of adjuvant pembrolizumab?
When I sit down with patients, I don’t try to scare them off it, but I also tell them that some patients will have irreversible adverse events [AEs]. Roughly 4% of patients will have adrenal insufficiency, and 1% of patients will get type 1 diabetes.3,7 There were no deaths on the trial, but the truth is some patients will die if you’re going to treat [patients] worldwide. Some patients will not do well. I quote them a 1% chance of death with immune checkpoint inhibition.
Some of the classic AEs [include]…hypothyroidism, which nobody is afraid of, but adrenal insufficiency is much harder. Type 1 diabetes is a life-changing event. Remember, 50% to 70% [of patients in the adjuvant setting may] never have needed immunotherapy. In my practice, patients already come with a mind-set. I give them the information, and some patients will…[go to other oncologists] if I tell them I’m not going to do it. If they want it, I’m going to give it.
Some patients are very hesitant and…say, “I just want to be followed.” That’s fine too. To me, active surveillance is still adequate for patients who are hesitant about… [pembrolizumab]. If patients absolutely want it, I give it, but I try to give a balanced discussion about where the field is.
REFERENCES
1.NCCN. Clinical practice guidelines in oncology. Kidney cancer, version 4.2023. Accessed May 4, 2023. https://bit.ly/2TAx1m3
2. Motzer RJ, Ravaud A, Patard JJ, et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results. Eur Urol. 2018;73(1):62-68. doi:10.1016/j.eururo.2017.09.008
3.Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683-694. doi:10.1056/NEJMoa2106391
4.Pal SK, Uzzo R, Karam JA, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2022;400(10358):1103-1116. doi:10.1016/S0140-6736(22)01658-0
5.PROSPER: Phase III randomized study comparing perioperative nivolumab versus observation in patients with renal cell carcinoma (RCC) undergoing nephrectomy (ECOG-ACRIN EA8143). J Clin Oncol. 2020;38(suppl 15):TPS5101. doi:10.1200/JCO.2020.38.15_suppl.TPS5101
6.Motzer RJ, Russo P, Grünwald V, et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial. Lancet. 2023;401(10379):821-832. doi:10.1016/S0140-6736(22)02574-0
7.Powles T, Tomczak P, Park SH, et al;KEYNOTE-564 Investigators. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(9):1133-1144. doi:10.1016/S1470-2045(22)00487-9
8.Choueiri TK, Tomczak P, Park SH, et al. Pembrolizumab as post nephrectomy adjuvant therapy for patients with renal cell carcinoma: results from 30-month follow-up of KEYNOTE-564. J Clin Oncol. 2022;40(suppl 6):290-290. doi:10.1200/JCO.2022.40.6_suppl.290
9.Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595(7867):432-437. doi:10.1038/s41586-021-03642-9

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen