Roundtable Roundup: Multiple Myeloma
In separate, live virtual events, Jonathan L. Kaufman, MD, and Morie A. Gertz, MD, MACP, discussed concerns about using chimeric antigen receptor T-cell therapy for a patient with relapsed/refractory multiple myeloma who is experiencing rapid disease progression.
CASE SUMMARY
Eight years ago, a 63-year-old man received a diagnosis of multiple myeloma (IgGκ). He lived in a rural community. Recently, he presented with penta-refractory disease progression after 4 prior lines of therapy that included autologous stem cell transplant, 2 proteasome inhibitors, 2 immunomodulatory drugs, and 1 anti-CD38 antibody. He had hypertension controlled with lisinopril. His ECOG performance status was 1.
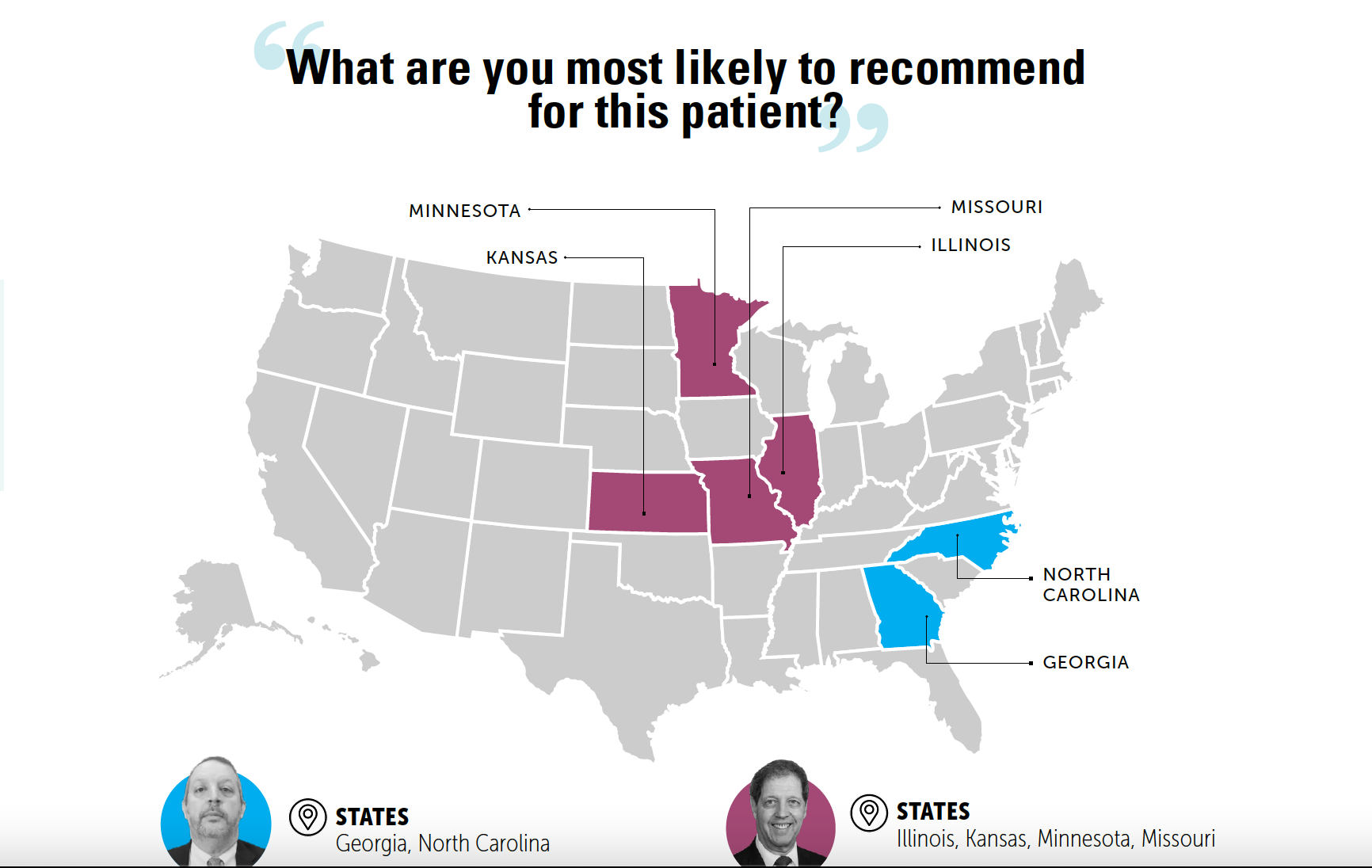
Professor, Department of Hematology and Medical Oncology
Emory University School of Medicine
Medical Director and Section Chief, Ambulatory Infusion Centers
Winship Cancer Institute of Emory University
Atlanta, GA

Morie A. Gertz, MD, MACP
Hematologist/Oncologist
Chair, General Internal Medicine
Mayo Clinic
Rochester, MN

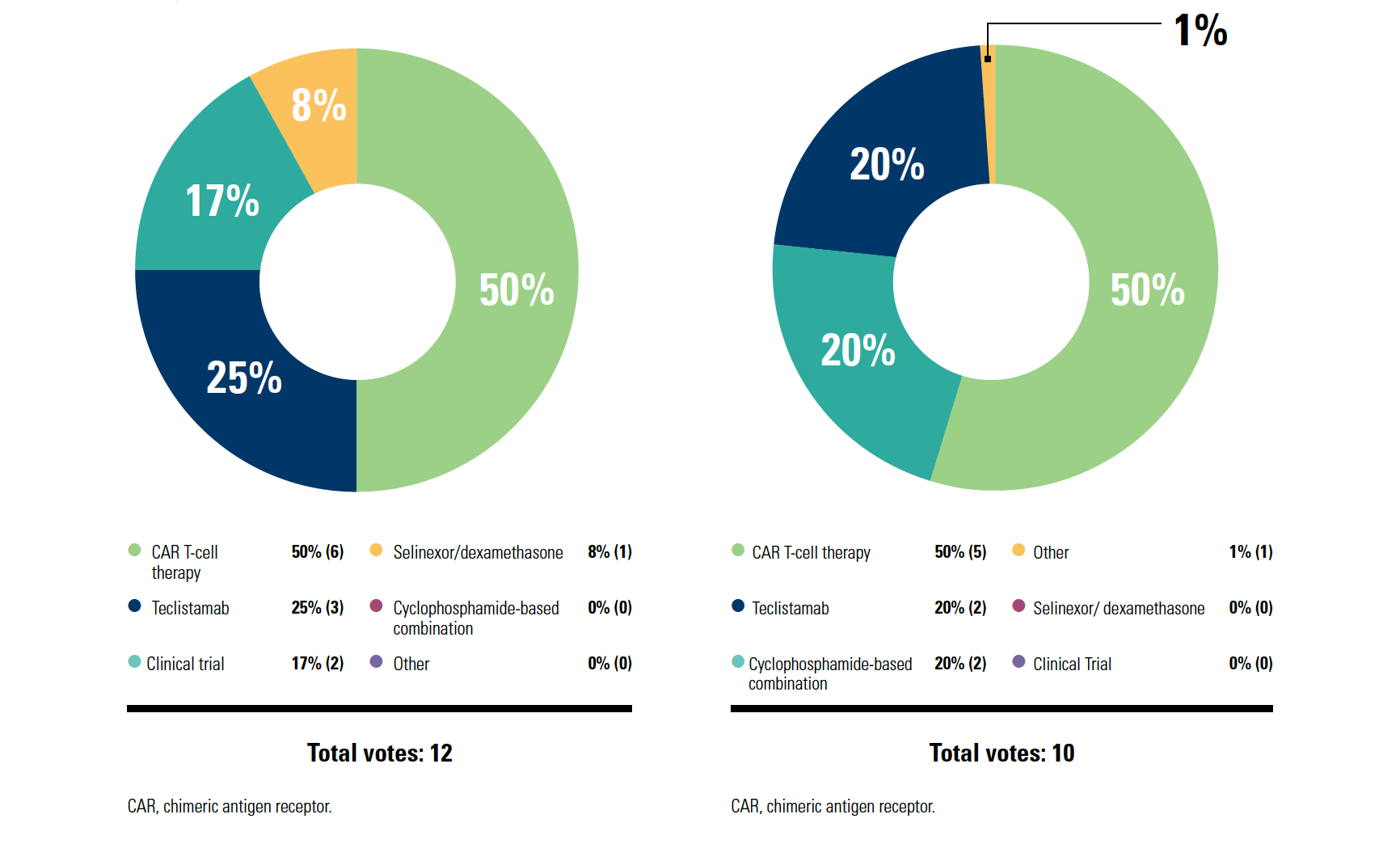
KAUFMAN: If I think about getting CAR T-cell therapy, if I think you as a patient need CAR T cells today, the best-case scenario is it happens in 3 or 4 months. That’s where we are today. [I hope] a year from now, we won’t be there…. It’s the logistics; although ideally maybe we like the efficacy, and we like the one-and-done nature of it—patients can come back to you and don’t have to have continuous therapy—it’s just not happening yet.
The way I think about it, for the patients with rapidly progressing disease, there’s no way that you can get lucky to have CAR T-cell therapy in [those] patients; you need bridging therapy. Then the more indolent relapse, where you see the relapse coming and you don’t stop therapy, that’s the patient who maybe can get a CAR T-cell slot 3 months from now.
Patient preference is important [for choosing next-line therapy]. When I talk to patients about it, some patients love the idea of one-and-done. Other patients don’t like how intense CAR T-cell therapy is and are OK with the ongoing therapy; they don’t like getting T cells collected, waiting the month, all those things. [Then there’s the] convenience logistics about getting it done—going to an academic center, getting the CAR T cells, coming back, and living a long, beautiful life. Performance status is critical. The way I lean is that [for patients with] better performance status, I can wait a bit and maybe that patient is right for CAR T cells.
GERTZ: Most CAR T-cell therapy mandates anywhere [state] that your [patient stays] 1 month at a medical center. So there’s no commute; they’re there, they get a hotel room, etc. CAR T-cell therapy would be a reasonable option, although for me, cyclophosphamide is a very common bridging therapy once they’re accepted.
Supply chain has limited access to commercial CAR T-cell therapy…it’s a real pain. Patients can sometimes wait months, and many of them don’t have months to wait. A significant cause of death is death on the waiting list, waiting to get availableCAR T-cell therapy.
The National Comprehensive Cancer Network guidelines [recommend] bendamustine, cyclophosphamide, or bendamustine combinations and then, [after 4 prior lines of therapy], CAR T cells, an antibody-drug conjugate, and the bispecific antibody teclistamab [Tecvayli]. You still have the option to use selinexor [Xpovio]. At this point, reimbursement hasn’t been a big problem, but it’s a hard drug to use.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More