Immunotherapy Combinations and Monotherapy Considered in a Patient With Metastatic Melanoma
During a Targeted Oncology™ Case-Based Roundtable™ event, Sajeve S. Thomas, MD, discussed key trials of immunotherapy in terms of safety and efficacy for patients with metastatic melanoma.
Sajeve S. Thomas, MD
Medical Oncologist and Hematologist
Orlando Health Cancer Institute
Assistant Professor
Florida State University College of Medicine
University of Central Florida College of Medicine
Orlando, FL

CASE SUMMARY
- 78-year-old man has a history of stage III melanoma.
- Patient underwent surgical resection 12 years prior.
- Lymph node dissection (LND) was positive for nodal involvement.
- Patient declined complete LND and adjuvant systemic therapy.
- Patient remained active since his surgery and maintained regular follow-up.
- On routine follow-up, the patient now presented with moderate asthenia that limited his daily activities, without other relevant clinical symptoms.
- ECOG performance status: 1
- Physical examination was unremarkable.
- Notable laboratory findings: lactate dehydrogenase (LDH) level of 380 UI/L (reference range, 110-240 UI/L)
- Full-body CT scan revealed the presence of pulmonary and hepatic nodules but no evidence of brain metastases.
- The patient underwent core-needle biopsy of the largest hepatic lesion in segment IVb without any complications.
- Pathology revealed metastatic melanoma.
- Mutation testing: BRAF negative
TARGETED ONCOLOGY: Can you discuss dual therapy vs monotherapy first-line options for stage IV melanoma?
THOMAS: CheckMate 067 [NCT01844505] was a phase 3 study looking at nivolumab [Opdivo] with or without ipilimumab [Yervoy] com-pared with ipilimumab alone.1 The KEYNOTE-006 trial [NCT01866319] investigated pembrolizumab [Keytruda] vs ipilimumab as the comparator arm.2 Then there was the new study of RELATIVITY-047 [NCT03470922] with nivolumab and relatlimab [Opdualag] vs nivolumab alone.3 We had nice 6- to 7-year follow-up data for CheckMate 067 with the ipilimumab/nivolumab or nivolumab vs ipilimumab compared with the RELATIVITY-047 [trial’s] relatively short follow-up in the range of 2 to 3 years.
Prior to 2011, IL-2 had a 1 in 20 response rate, and when we had ipilimumab monotherapy, it was a 1 in 5 response rate. I had patients who just received ipilimumab. [They] never went on to get other therapy, 3 or 4 doses, and here they are 10 years later in remission and doing fine. Then PD-1 therapy moved that bar to about [a] 1 in 3 response rate. Response rates with ipilimumab/nivolumab were 58% and 43% in the nivolumab/relatlimab arm.1,4
Q: What were the survival results for these regimens?
The median progression-free survival [PFS] for ipilimumab/ nivolumab was 11.5 months vs nivolumab alone at 6.9 months [HR, 0.79; 95% CI, 0.65-0.97].1 Pembrolizumab’s median PFS was 8.4 months [HR, 0.57; 95% CI, 0.48-0.67], and relatlimab/nivolumab’s [median PFS] was 10.2 months [HR, 0.78; 95% CI, 0.64-0.94].2,3 So with dual inhibition, you are getting better PFS compared with monotherapy. There was a 6.5-year PFS rate with ipilimumab/nivolumab of 34%. But in the nivolumab arm, there was a 6.5-year PFS of 29%, and that is for monotherapy. That is why I always point out that for patients who have low-volume disease, no brain metastases, who are doing relatively well, especially if they [have] BRAF wild-type [disease], they may do fine. [One-third] of these patients may do fine with just monotherapy.
If you look at the overall survival [OS] with ipilimumab/ nivolumab at 5 years, it was 52%, and at 6.5 years, [it was] 49%.1 With nivolumab, it was 44% [at 5 years] and 42% [at 6.5 years], so not that far off. The design of the study was not to compare nivolumab with ipilimumab/nivolumab. It was nivolumab plus or minus ipilimumab vs ipilimumab alone. We do not yet have long-term data on relatlimab/nivolumab.
Q: Can you describe the immune-related adverse events (AEs) seen with immunotherapy in these patients with melanoma?
Immune-related AEs are different from a lot of the chemotherapy-related AEs we have to deal with. For most patients, the first couple of doses are not too bad with monotherapy. I tell my patients that 70% of the time it’s a walk in the park, 25% of the time there are mild to moderate symptoms, and 5% of the time patients can get severe related issues [such as] colitis or pneumonitis: 1:100 with adrenal insufficiency and 1:1000 with type 1 diabetes where they are taking insulin. Compare that with using ipilimumab/ nivolumab, where 40% can do relatively OK, 40% have mild to moderate symptoms, and 20% have severe related issues.
There is 1 patient we included in the phase 2 study of a PD-1 therapy plus talimogene laherparepvec [Imlygic].5 This patient, who had been on the protocol for 2 years, came in for the last dose. She [had hypoxia], was in the intensive care unit for about 2 weeks, and had 2 months of rehabilitation in addition to home oxygen, but she recovered. So you have to be careful with these AEs. They can be on the drug for some time, but out of the blue, 8 months or 2 years later, these things can pop up.
The other things that can happen, especially if they are off therapy, are endocrinopathy-related issues: hyperthyroidism, adrenal insufficiency, hypophysitis, and type 1 diabetes. These things can happen as you stop therapy. I would still recheck the laboratory results periodically as you check their scans over time. I have a low threshold to use steroids at 0.5, 1, or 2 mg/kg, depending on the severity of the symptoms.
A lot of times I will give them a bolus dose at the infusion center. Then we give a 60- or 40-mg taper over 2 to 4 weeks, depending on what symptoms they have and the severity of the symptoms that we are dealing with, hold their therapy, and then restart therapy and potentially restart their PD-1 therapy if they had a problem with dual therapy in the beginning. Then think about your plan B, because eventually you are going to give infliximab [Remicade] or vedolizumab [Entyvio] for colitis issues or mycophenolate [CellCept] for steroid-refractory hepatitis issues.
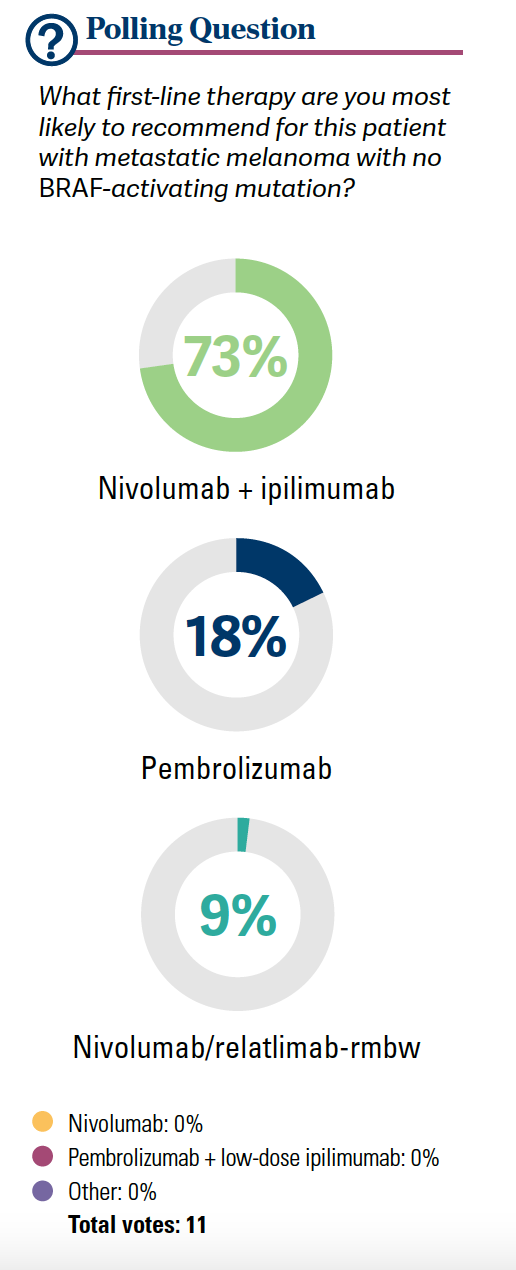
Seventy-three percent of the participants gave ipilimumab/nivolumab [in the poll]. I imagined there was going to be a bit more colitis [that was] steroid refractory. If they [have] steroid-refractory [disease], rule out for infectious [disease], but most often we will give infliximab 5 mg/kg in a 1-time dose, and that is it. We are consulting with colleagues to consider other possibilities, but this is something that we will see a lot more of over time.
Q: Can you compare the safety data in CheckMate 067 with those in RELATIVITY-047?
[Results from] the CheckMate 067 study [looking at] ipilimumab/nivolumab had a rate of grade 3 or 4 AEs at 55% [with the combination] and 16% with nivolumab alone.1 If look at RELATIVITY-047, with relatlimab/nivolumab, grade 3 or 4 AE rates were at 21%.3 There was a bit of hepatitis, a bit more adrenal insufficiency, and a touch of myocarditis. But if you look at CheckMate 067, [one-third] of patients who had grade 3 or 4 issues had lipase and amylase elevations, which I am pretty sure most physicians are not checking unless patients have symptoms of pancreatitis or they’re on protocol.1 And there’s another set of patients who have aspartate aminotransferase and alanine transaminase elevations. They are not necessarily symptomatic from that, as opposed to pneumonitis, dermatitis, or colitis. Most of this tends to be more metabolic. But we can see any part of the body affected [when] giving immunotherapy, either dual or monotherapy. It’s something we have to deal with, and [you have to deal with] more of it if you are doing dual inhibition.
Q: What were the design, efficacy, and AEs in the KEYNOTE-006 trial?
The phase 3 KEYNOTE-006 study of pembrolizumab vs ipilimumab is an old study.2 All patients had metastatic melanoma. In this trial, there was weight-based dosing of pembrolizumab 10 mg/kg every 2 weeks, pembrolizumab 10 mg/kg every 3 weeks, or what used to be first-line therapy for a brief time: ipilimumab 3 mg/kg [every 3 weeks] for 4 doses.
If a patient got pembrolizumab, whether they did every 2 or 3 weeks, the median OS was not reached.2 The OS HR was 0.68 [95% CI, 0.53-0.87; P < .01]. For ipilimumab, the median OS was 16 months [95% CI, 13.5-22.0]. For those who had pembrolizumab every 2 or 3 weeks, the complete response [CR] rate was 12% and 13% and partial responses were 25% and 23%, respectively. The overall response rate was 36% compared with ipilimumab, where you have lower single-digit responses. The median duration of response was not reached. So for those responders, even with monotherapy, these patients can do extremely well. Even for the ipilimumab responders, the median duration of response was not reached. I had patients who had ipilimumab alone, way back in the day, and they are doing fine today.
AEs were [approximately] 17% for [grade 3/4] AEs with pembrolizumab at 2 or 3 weeks, and the main AEs were diarrhea, itchy skin, dermatitis, and hypothyroidism.2 Any-grade hypothyroidism was 11% for the every-2-weeks group, 8% for the every-3-week[s] group, and 1% [ for those] on ipilimumab, although with ipilimumab we probably see a bit more adrenal insufficiency and hypophysitis.
Can you discuss the details of the CheckMate 067 trial?
The CheckMate 067 study was not a study comparing ipilimumab/nivolumab with nivolumab.1 It [was] nivolumab with or without ipilimumab compared with ipilimumab alone as the comparator arm. There were 900 patients with unresectable metastatic melanoma, stratified by BRAF status, stage, and PD-1 expression. They continued until progression [or] unacceptable toxicity or according to patient preferences. Secondary end point was the overall response rate. Patients who had autoimmune [disease], ocular disease, or active brain metastases were excluded.
Median PFS was 11.5 months with ipilimumab/nivolumab, 6.9 months with nivolumab, and 2.9 months with ipilimumab.1 Median OS was favorable for the ipilimumab/nivolumab arm at 72.1 months vs 36.9 months for nivolumab vs 19.9 months for ipilimumab. But what’s that delta between ipilimumab/ nivolumab and nivolumab? If we are going to make that comparison in terms of PFS and OS, you are talking about a 3% to 6% difference. There is a tail on the Kaplan-Meier curve in those who got monotherapy. For ipilimumab/nivolumab, the OS rate was seen in about half of patients. With nivolumab, it was about 42%, so about a 6% to 7% difference there with nivolumab compared with ipilimumab/nivolumab.
If you look at response rate, the most important thing is [that] the immune system shrinks tumors by the time you get to your next CT scan. The objective response rate was 58% with ipilimumab/nivolumab, 45% with nivolumab— which is a bit better than some of the prior studies—and with ipilimumab it was [approximately] 19%. The median duration of response, whether the patient got ipilimumab/ nivolumab or nivolumab monotherapy, was not reached. The rate of grade 3 or 4 events with ipilimumab/nivolumab was approximately 60%, nivolumab was 24%, and ipilimumab monotherapy was [28%].
Q: What were the design and demographics in the RELATIVITY-047 trial?
The phase 3 RELATIVITY-047 study was a global, randomized, double-blinded study of [unmanaged], unresectable metastatic melanoma, stratified by LAG-3 expression, PD-L1 expression, BRAF mutation, and stage.3 It was a 1:1 randomization to the combination of nivolumab and relatlimab at a fixed dose every 4 weeks vs a fixed dose of nivolumab every 4 weeks. The primary end point was PFS, and secondary end points were OS and response rates.
If you look at the demographics, you have about 26% of patients with M1a disease—skin only, lymph node only, low LDH level, M1b disease in lung only. [Approximately] 35% to 40% of these patients had M1c disease, so this is nonpulmonary visceral metastasis, probably liver, bone, that kind of thing. M1d disease would be brain metastases [that were managed] if they were part of the study. All had good performance status. LDH level was elevated above normal in 36% of patients and significantly higher in 9% of patients. Approximately 8% had prior neoadjuvant or adjuvant therapy, and most of these patients had interferon. LAG-3 expression was more than 1% in 75% of patients. PD-L1 expression more than 1% was seen in 41% of patients. BRAF mutation was seen in nearly 40% of these patients. M1 [stage] with high LDH level was seen in about 34% of these patients.
Q: In terms of efficacy, how did these patients with melanoma respond when receiving nivolumab/relatlimab?
If you look at the primary end point of median PFS, for those who got the combination, it was 10.2 months.3 For those who got nivolumab, PFS was 4.6 months [HR, 0.78; 95% CI, 0.64- 0.94]. The 2-year PFS rate was 38.5% with the combination and 29% with nivolumab, which is on par with what we have seen with prior monotherapy. But the dual therapy was better compared with the monotherapy arm.
In the subgroup analysis of PFS, I think there are some interesting data.... Today, I do not think [LAG-3 testing] is commercially available, though we do it [in] other trials. I do not think you can test it [outside] of the trial. But with LAG-3 expression above 1%, patients did better with dual therapy. If it was less than 1%, they did better.
What I find interesting is this PD-L1 expression, because if patients had PD-L1–positive disease, they did not do as well with the combination.3 It looks like it was even [between the 2 arms] if they had PD-L1–positive disease. If they had PD-L1–negative disease, the nivolumab/relatlimab arm did better compared with the nivolumab [arm]. That is how I would interpret that.
If patients had BRAF-mutated disease, it was favorable, and wild-type [disease] was also favorable. Regardless of staging, it favored nivolumab/relatlimab compared with nivolumab alone. [If we look at] LDH level, performance status, burden of the disease, and sites of metastases, they all seem to favor the nivolumab/relatlimab group. The OS rate is more descriptive, not a statistical difference. The combination seemed to have done better [median OS, not reached vs 34.1 months]. There was a 2-year OS rate of 63.7% for nivolumab/relatlimab vs 58.3% for nivolumab and a 3-year OS rate of 55.8% vs 48.8%, respectively.3
The response rate with the combination of nivolumab/ relatlimab was 43.1%.3 For those who got nivolumab, it was 32.6%. There were more CRs and partial responses [in the nivolumab/relatlimab arm], and stable disease was about equal in each group. The disease control rate was 62.8% vs 50.7%, respectively. The duration of response, whether they got nivolumab/relatlimab or nivolumab alone, was not reached.
Q: What was the toxicity profile seen in RELATIVITY-047?
Treatment-related AEs of grade 3 or 4 in the nivolumab/ relatlimab group were at 21.1% vs the nivolumab group at 11.1%.3 We have some non–immune-related AEs that we can potentially see a little more of.
In terms of treatment-related deaths, for all patients, there is something that we have seen even with PD-1 therapy: hemophagocytic lymphohistiocytosis. I had a patient like that [in the past] year on PD-1 monotherapy treatment, with high-grade fevers, anemia, thrombocytopenia. And we found organ evidence of hemophagocytosis, pneumonitis, multiorgan failure, sepsis, myocarditis. These are things we are going to worry about in all patients. That is why it is important that we pay attention to the AEs and start steroids and immunosuppressive therapy if they are developing signs of immune-related issues.
I will point out that for grade 3/4 adrenal insufficiency, there are 6 patients in the nivolumab/relatlimab group vs 0 in the nivolumab group.3 Although if you look at PD-1 monotherapy, it is typically about a 1:100 risk overall. If you look at grade 3 or 4 hepatitis, there were…15 patients or 4.2% for the combination vs 6 patients or 1.7% for nivolumab. All-grade hepatitis was 5.9% vs 3.1%, respectively. There was any-grade myocarditis in 1.7% with the combination vs 0.6% with nivolumab. So as part of protocols and including a lot of other protocols, we would often do troponin [level] monitoring.
References
1. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 2022;40(2):127-137. doi:10.1200/JCO.21.02229
2. Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239-1251. doi:10.1016/S1470-2045(19)30388-2
3. Tawbi HA, Schadendorf D, Lipson EJ, et al; RELATIVITY-047 Investigators. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24-34. doi:10.1056/NEJMoa2109970
4. Long GV, Hodi FS, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in previously untreated metastatic or unresectable melanoma: overall survival and response rates from RELATIVITY-047 (CA224-047). J Clin Oncol. 2022;40(suppl 36):360385. doi:10.1200/JCO.2022.40.36_suppl.360385
5. Sun L, Funchain P, Song JM, et al. Talimogene laherparepvec combined with anti-PD-1 based immunotherapy for unresectable stage III-IV melanoma: a case series. J Immunother Cancer. 2018;6(1):36. doi:10.1186/s40425-018-0337-7

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More