Choueiri Looks at the Use of Newer Regimens in Advanced RCC
During a Targeted Oncology Case-Based Roundtable event, Toni K. Choueiri, MD, discussed treatment options using immune checkpoint inhibitors in patients with advanced renal cell carcinoma.
Toni K. Choueiri, MD
Director, Lank Center for Genitourinary Oncology, Dana-Farber Brigham Cancer Center

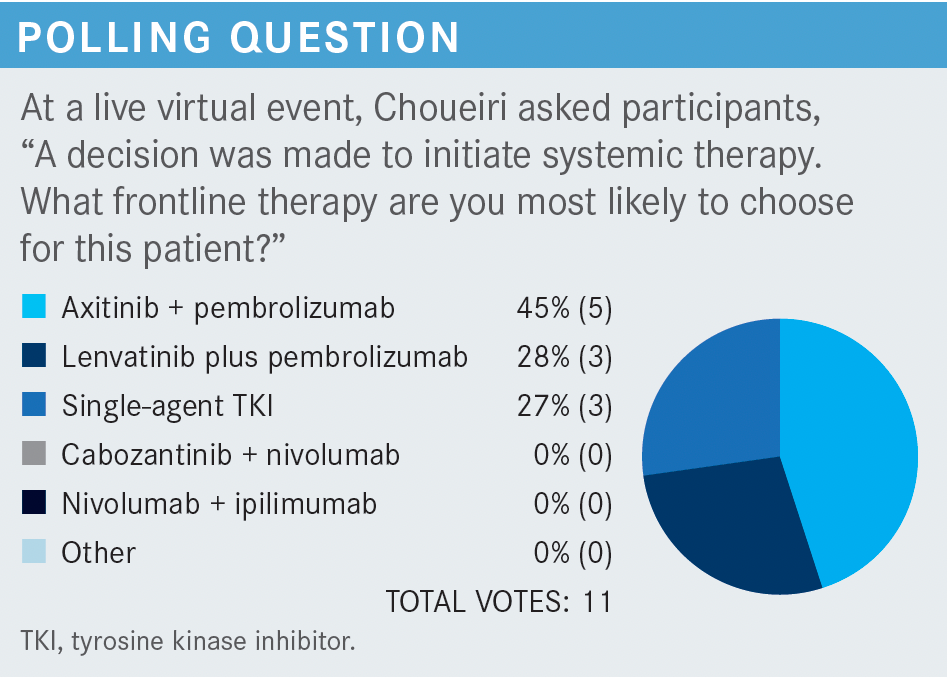
Targeted OncologyTM: What treatment options exist for relapsed or stage IV clear cell RCC in patients like this one with poor or intermediate risk?
CHOUEIRI: The guidelines of the National Comprehensive Cancer Network [NCCN] recommend axitinib [Inlyta] plus pembrolizumab [Keytruda], cabozantinib [Cabometyx] plus nivolumab [Opdivo], ipilimumab [Yervoy] plus nivolumab, and lenvatinib [Lenvima] plus pembrolizumab, all supported by category 1 data. [Single-agent] cabozantinib is another preferred regimen, but it is not supported by category 1 data.1
The combination of axitinib plus avelumab [Bavencio], listed among the “other recommended” regimens,1 is FDA approved,2 though avelumab [has not yet been shown to confer] an overall survival [OS] benefit.3 Single-agent pazopanib [Votrient] and single-agent sunitinib [Sutent] are also listed as “other recommended regimens,” [useful] for patients who can’t receive the doublets.1
Among the regimens listed as “useful in certain situations” is [single-agent] axitinib,1 to be used if you need a drug with a very, very short half-life. Other regimens in this category are high-dose IL-2,1 which is still not [used] at our hospital, and temsirolimus [Torisel],1 to be used in situations where immuno-oncology [IO] and VEGF-targeted treatments are contraindicated.
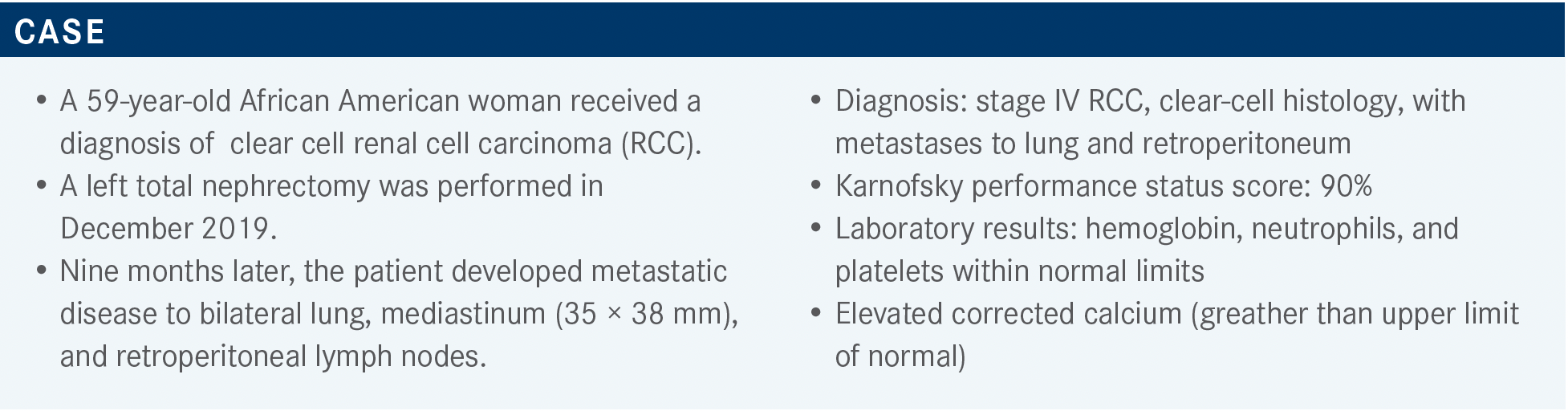
What data support the use of regimens that feature immune checkpoint inhibitors?
[I can describe] 4 FDA-approved regimens that showed an OS benefit. Ipilimumab plus nivolumab was [approved for patients of] intermediate and poor risk.4 In comparison with studies of axitinib plus pembrolizumab [KEYNOTE-426 (NCT02853331)],5-7 cabozantinib plus nivolumab [CheckMate 9ER (NCT03141177)],8-10 and lenvatinib plus pembrolizumab [CLEAR (NCT02811861)],11-13 the study of ipilimumab plus nivolumab [CheckMate 214 (NCT02231749)] had the longest median follow-up [55 months].14
All these [regimens benefited] OS, progression-free survival [PFS], and objective response rate [ORR]. However, because there were differences in median follow-up and because the study populations had different baseline characteristics, like the risk category distribution and the percentage of nephrectomy recipients, it’s difficult to compare the study results.

Can you expand on the data from the CLEAR trial of frontline lenvatinib plus pembrolizumab in patients with advanced RCC?
In that trial, lenvatinib plus pembrolizumab was compared with sunitinib and with lenvatinib plus everolimus [Afinitor],11 a regimen approved [for second- or third-line use] after RCC progression on sunitinib.15
[In the first arm of this study], lenvatinib16 was administered at a dosage of 20 mg daily; pembrolizumab17 was administered at a dosage of 200 mg every 3 weeks.11 Be careful of the tyrosine kinase inhibitor [TKI] dosage [when combined with an] IO; often the TKI [is not given at the full dosage]. In the second arm, lenvatinib was administered at a dosage of 18 mg daily, and everolimus at a dosage of 5 mg daily. In the third arm, sunitinib was administered at a dosage of 5 mg daily for 4 weeks, followed by 2 weeks off. The primary end point in this study was PFS.11
The combination of lenvatinib plus pembrolizumab yielded the longest PFS [23.9 months; 95% CI, 20.8-27.7 months] with an HR of 0.39 vs sunitinib [95% CI, 0.32-0.49; P < .001]. Lenvatinib plus everolimus yielded a PFS of almost 15 months [HR vs sunitinib, 0.65; 95% CI, 0.53-0.80; P < .001]. PFS with sunitinib was 9.2 months and subgroup PFS analyses by sex, age, International Metastatic Renal Cell Carcinoma Database Consortium risk group, and others all favored the combination regimens over single-agent [sunitinib].11
In the analysis of OS, lenvatinib plus pembrolizumab performed best [HR vs sunitinib, 0.66; 95% CI, 0.49-0.88; P = .005]. [In contrast], for the non-IO combination of lenvatinib plus everolimus,18 the HR vs sunitinib for OS was 1.15 [95% CI, 0.88-1.50; P = .3].11
Another feature that differentiates the lenvatinib plus pembrolizumab combination is the ORR; [from among the 3 regimens, only lenvatinib plus pembrolizumab had an ORR that exceeded] 70%.11 The rate of complete response [CR] among the intermediate/poor population was 14%, and among the overall population the rate of CR was 16%.11,12 One feature of the lenvatinib16 plus pembrolizumab17 regimen that is common among VEGF-targeted or IO combinations was a very low rate of progressive disease [PD] as best response. In this study, the rate of PD among patients who got scans after 2 or 3 months was 5.4%.11 When a patient’s disease is [progressing] very fast, you want [to use] a VEGF-targeted or IO combination. [In contrast], the PD rate of patients on nivolumab plus ipilimumab [has been shown to be about] 20%,14 and sometimes you can’t afford that.
Finally, despite meeting the primary end point of PFS, as well as yielding a clinically relevant and significantly higher ORR, the lenvatinib plus everolimus combination didn’t get approval vs sunitinib [as frontline therapy] because it produced no OS [benefit].11 It would have been nice to have [this regimen as a frontline option] for those rare patients who have a contraindication for IO regimens.
What did the CLEAR trial show about the safety of lenvatinib plus pembrolizumab?
We have to learn how to manage TKIs [such as lenvatinib].16 I have many patients who cannot tolerate 20 mg per day of lenvatinib, so I reduce the dosage to 18, 14, or 10 mg per day. I have a patient who had a lot of [adverse] effects at a daily dose of 14 mg; his creatinine [level] went from 1.6 to 2.8 [mg/dL] because of diarrhea. We stopped the treatment, he got better, and then we restarted lenvatinib at 10 mg daily. As of today, his response is maintained.
Despite [the fact] that this regimen can cause toxicity, most of the toxicities tend to be of low grade. The high-grade toxicities, overall, are manageable. With pembrolizumab, you do have to [watch out for] proteinuria; in this study, about 7% of patients had proteinuria of grade 3.11
Hypertension is another concern,11 but we’re able to manage it. I usually start patients on an angiotensin-converting enzyme [ACE] inhibitor [untill the maximum dose then I switch] to a calcium channel blocker, then to a β-blocker. After trying these 3 treatments I send for consultation.
What are some practical considerations for physicians who use lenvatinib plus pembrolizumab?
The practical considerations are all [concerned with] the dosage. The recommended, but not mandated, dosage is [200 mg of pembrolizumab every 3 weeks] plus 20 mg of lenvatinib daily. The dose reduction scheme for lenvatinib is to reduce to 14, 10, and finally to 8 mg daily.16,17 I [usually] start at 20 or 18 mg; I’ve [had] some patients [with uncontrolled hypertension] start at 14 mg.
Usually, regardless of the response, pembrolizumab is stopped after 2 years and lenvatinib is continued as a single agent. But if you think the patient is benefiting from the pembrolizumab and the patient is not comfortable with stopping and the insurance is not calling on you to stop, it’s not at all wrong [to continue].
Now remember, when administered with everolimus, lenvatinib was given at a dosage of 18 mg daily.11 There’s a study of lenvatinib plus everolimus [NCT03173560], following treatment with sunitinib, [testing] 2 different dosages [of lenvatinib]: 18 mg and 14 mg. The 18-mg dosage performed slightly better, but I don’t believe the effect was statistically significant.19
What data support the use of frontline nivolumab plus cabozantinib in patients with advanced or metastatic RCC?
The CheckMate 9ER trial [NCT03141177] examined nivolumab plus cabozantinib, used as frontline therapy, in patients with metastatic clear cell RCC. The 651 patients were randomly assigned to receive either nivolumab, 240 mg every 2 weeks, plus cabozantinib, 40 mg daily, or sunitinib. The primary end point of CheckMate 9ER was PFS. The nivolumab plus cabozantinib combination [median PFS, 16.6 months; 95% CI, 12.5-24.9] outperformed sunitinib [median PFS, 8.3 months; 95% CI, 7.0-9.7] with an HR of 0.51 [95% CI, 0.41-0.64; P < .0001].8,20 We presented an update earlier this year [showing that] the median PFS increased to 17.7 months and the HR was stable at 0.54 [95% CI, 0.43-0.64].21
Across all subgroups, the HR was less than 1, favoring the nivolumab plus cabozantinib combination.8,20 The secondary end point of this study, OS, was also met. The HR for OS was 0.60 [99.89% CI, 0.40-0.89; P = .0010], a 40% decrease in the risk of death. After 2 years of follow-up, the HR increased to 0.66 [95% CI, 0.50-0.87], a 34% decrease in the risk of death.21
The ORR for the combination arm was 55.7% [95% CI, 50.1%-61.2%], which was double that of the single-agent sunitinib arm [P < .0001 for the between-group difference]. The rate of CR was double also [8% for the combination, 4.6% for the single agent].8,20 These data all suggest the benefit of nivolumab plus cabozantinib over sunitinib.
The dose of cabozantinib was reduced in 56.3% of patients, and cabozantinib was discontinued because of treatment-related adverse events [TRAEs] in 6.6%. Both nivolumab and cabozantinib were discontinued because of TRAEs in 3.1% of patients. Most of the treatment discontinuations were due to PD.20 Most of the AEs were of grade 1 and 2. Some of the higher-grade AEs were, not surprisingly, hypertension [11% for the combination arm], hand-foot syndrome [8% in both arms], and diarrhea [6% for the combination arm]. Most of the AEs can be managed.20
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 3.2022. Accessed December 7, 2021. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
2. FDA approves avelumab plus axitinib for renal cell carcinoma. FDA. Updated May 15, 2019. Accessed December 10, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-avelumab-plus-axitinib-renal-cell-carcinoma
3. Choueiri TK, Motzer RJ, Rini BI. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020 Aug;31(8):1030-1039. doi: 10.1016/j.annonc.2020.04.010
4. FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma. FDA. April 16, 2018. Accessed December 10, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-ipilimumab-combination-intermediate-or-poor-risk-advanced-renal-cell
5. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): results from 42-month follow-up of KEYNOTE-426. J Clin Oncol. 2021;39(suppl 15):4500. doi:10.1200/JCO.2021.39.15_suppl.4500
6. Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563-1573. Published correction appears in Lancet Oncol. 2020;21(12):e553.
7. FDA approves pembrolizumab plus axitinib for advanced renal cell carcinoma. FDA. Updated April 22, 2019. Accessed December 10, 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-plus-axitinib-advanced-renal-cell-carcinoma
8. Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
9. Apolo AB, Powles T, Burotto M, et al. Nivolumab plus cabozantinib (N+C) versus sunitinib (S) for advanced renal cell carcinoma (aRCC): outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. J Clin Oncol. 2021;39(suppl 15):4553. doi:10.1200/JCO.2021.39.15_suppl.4553
10. FDA approves nivolumab plus cabozantinib for advanced renal cell carcinoma. FDA. January 22, 2021. Accessed December 11, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-cabozantinib-advanced-renal-cell-carcinoma
11. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
12. Grünwald V, Powles T, Kopyltsov E, et al. Analysis of the CLEAR study in patients (pts) with advanced renal cell carcinoma (RCC): depth of response and efficacy for selected subgroups in the lenvatinib (LEN) + pembrolizumab (PEMBRO) and sunitinib (SUN) treatment arms. J Clin Oncol. 2021;39(suppl 15):4560. doi:10.1200/JCO.2021.39.15_suppl.4560
13. FDA approves lenvatinib plus pembrolizumab for advanced renal cell carcinoma. FDA. Updated August 11, 2021. Accessed December 10, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-plus-pembrolizumab-advanced-renal-cell-carcinoma
14. Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079. doi:10.1136/esmoopen-2020-001079
15. Lenvatinib in combination with everolimus. FDA. Updated May 16, 2016. Accessed December 10, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/lenvatinib-combination-everolimus
16. Lenvima. Prescribing information. Eisai Inc; 2021. Accessed December 10, 2021. https://www.lenvima.com/-/media/Project/EISAI/Lenvima/PDF/prescribing-information.pdf
17. Keytruda. Prescribing information. Merck Sharp & Dohme Corp; 2021. Accessed December 11, 2021. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
18. Afinitor. Prescribing information. Novartis Pharmaceuticals Corporation; 2021. Accessed December 11, 2021. https://www.novartis.us/sites/www.novartis.us/files/afinitor.pdf?irmasrc=ONCWB0043&source=01030
19. Pal SK, Puente J, Heng DYC, et al. Phase II trial of lenvatinib (LEN) at two starting doses + everolimus (EVE) in patients (pts) with renal cell carcinoma (RCC): results by independent imaging review (IIR) and prior immune checkpoint inhibition (ICI). J Clin Oncol. 2021;39(suppl 6):307. doi:10.1200/JCO.2021.39.6_suppl.307
20. Choueiri TK, Powles T, Burotto, et al. Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: first results from the randomized phase III CheckMate 9ER trial. Ann Oncol. 2020;31(suppl 4):S1159. doi:10.1016/j.annonc.2020.08.2257
21. Motzer RJ, Choueiri TK, Powles T, et al. Nivolumab + cabozantinib (NIVO+CABO) versus sunitinib (SUN) for advanced renal cell carcinoma (aRCC): outcomes by sarcomatoid histology and updated trial results with extended follow-up of CheckMate 9ER. J Clin Oncol. 2021;39(suppl 6):308. doi:10.1200/JCO.2021.39.6_suppl.308

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen