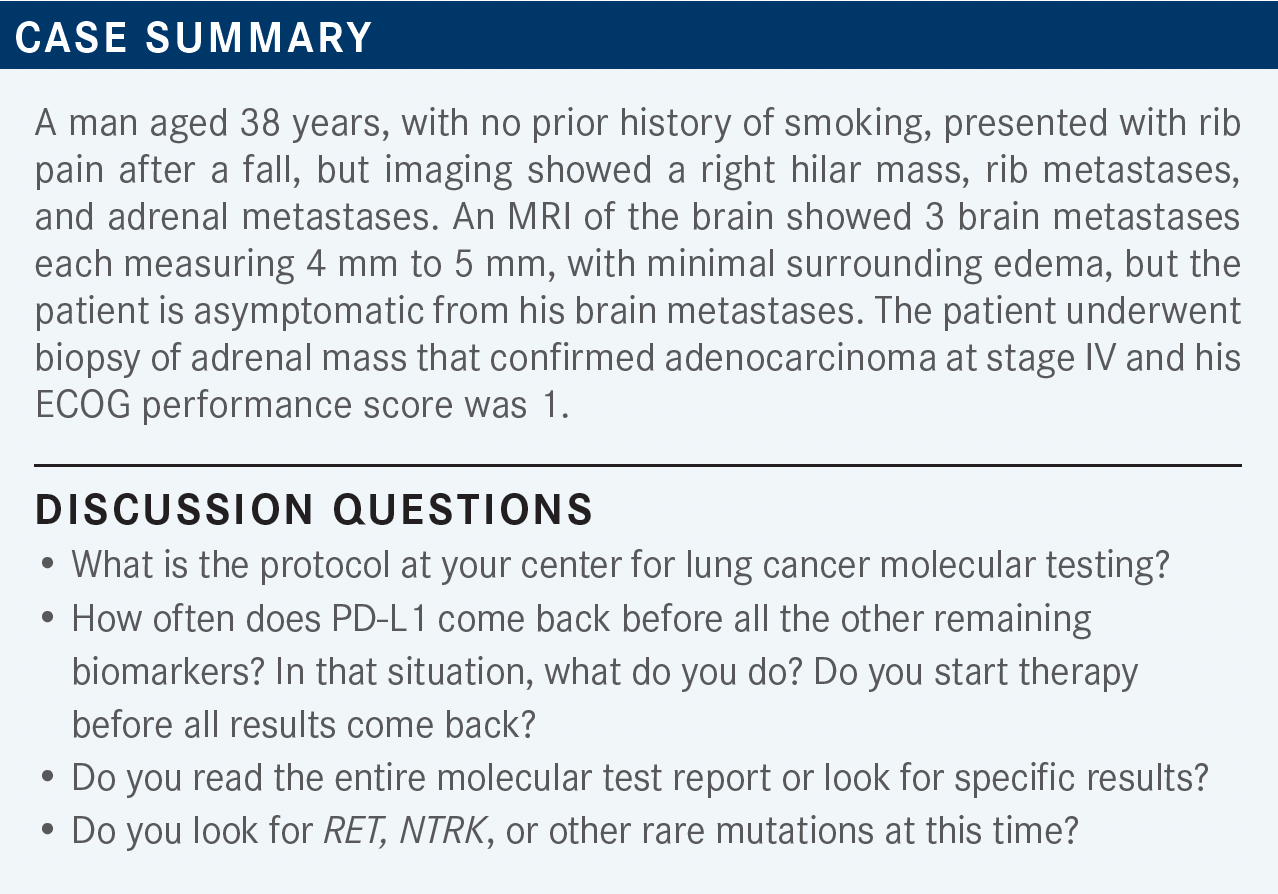
Socinski Evaluates Rationale for Treatment Options in Patients With Rare Driver Mutations in Lung Cancer
During a Targeted Oncology Case-Based Roundtable event, Mark A. Socinski, MD, discussed how to approach treating patients with lung cancer with rare mutations that can be targeted by certain treatments.
Mark A. Socinski, MD (Moderator)
Executive Medical Director
AdventHealth Cancer Institute
Member, Thoracic Oncology Program
Orlando, FL


CARTWRIGHT: Usually I do molecular testing on everybody, [and it’s] typically next-generation sequencing [NGS]—typically Foundation or something like that.
RAEZ: Because there is no reflex testing [at my practice], unfortunately, testing tends to be ordered by the medical oncologist, and [then we] wait 3 or 4 weeks for the tissue. I think the best solution is to do the liquid biopsy up front. Then you can get the results that you need and then you don’t have to wait too long [while the patient is still in] therapy for 4 weeks [awaiting their NGS] testing.
SOCINSKI: How many of you are also doing concurrent plasma-based testing while you’re waiting for the tissue? [That’s] assuming your tissue procurers get adequate samples for you to do tissue-based testing, which can sometimes be an issue. For me, I do tissue and blood [testing] on everybody, and I think it’s so important to find a driver mutation. One of the greatest things we can do as oncologists is find a molecular alteration [for which] there’s an effective targeted therapy.
CARTWRIGHT: I order [both types of tests] on pretty much everybody. Like you said, you want complete results, and [so the more the better].
RAEZ: According to the statistics, 17% of the time, if the blood is enough for us, [we can] make the decision in about 8 days, while the tissue tests take 3 weeks. That’s why we order both, because the blood is not perfect, as you said, and the [blood tests] don’t catch a lot of the fusions. So, we still need the tissue sometimes.
LI: We do the same, for [patients with lung cancer] especially. [When the disease] is metastatic, it so seldom needs a [traditional survey] so we usually get a needle biopsy. [In this patient case], the sample was not big enough, and usually it’s not enough.
We then send the liquid biopsy, in combination with the tissue biopsy, and in case there isn’t a report with the tissue biopsy then we have a core biopsy, which we usually have a better chance [of getting results].

SOCINSKI: I’m getting the sense that most of you do not necessarily have reflex testing at your institution. You must initiate it if it wasn’t initiated by someone else.
RAEZ: One problem that we have is that [pathology is advancing more with a good rate of success]. They are creating more and more immunochemistry markers, and [checking for them] takes a lot of tissue…someone like you knows that very well, [but] I don’t know if everybody knows that. Cause they know in lung cancer they don’t only do PD-L1 [testing], they do METs and they do CK7 [as well].
SOCINSKI: I would also say the NGS information is more important than the [immunohistochemistry] information. Right?
RAEZ: Well, the other problem is that [those tests] don’t take ownership of the reflex testing, so they are happy doing their job with these chemistries. And then, they don’t worry, I guess, about the NGS.
SOCINSKI: It requires a lot of good communication between you and the pathologist so that they know what our priorities are in these situations. What about PD-L1 testing?
LI: PD-L1 testing usually requires more tissue. [The tissue you get with] a needle biopsy, especially if it’s [done with] a bronchoscopy, is not sufficient for PD-L1 [testing and causes] frequent delay.
SOCINSKI: Most often, remember, all the studies were cyclin D1, [a protein required for disease progression] initially with core biopsies. So, a lot of our diagnoses are made on [tissue retrieved with] fine needle aspiration [FNA]. A robust FNA is great for molecular testing, but it may not be great for PD-L1 testing.
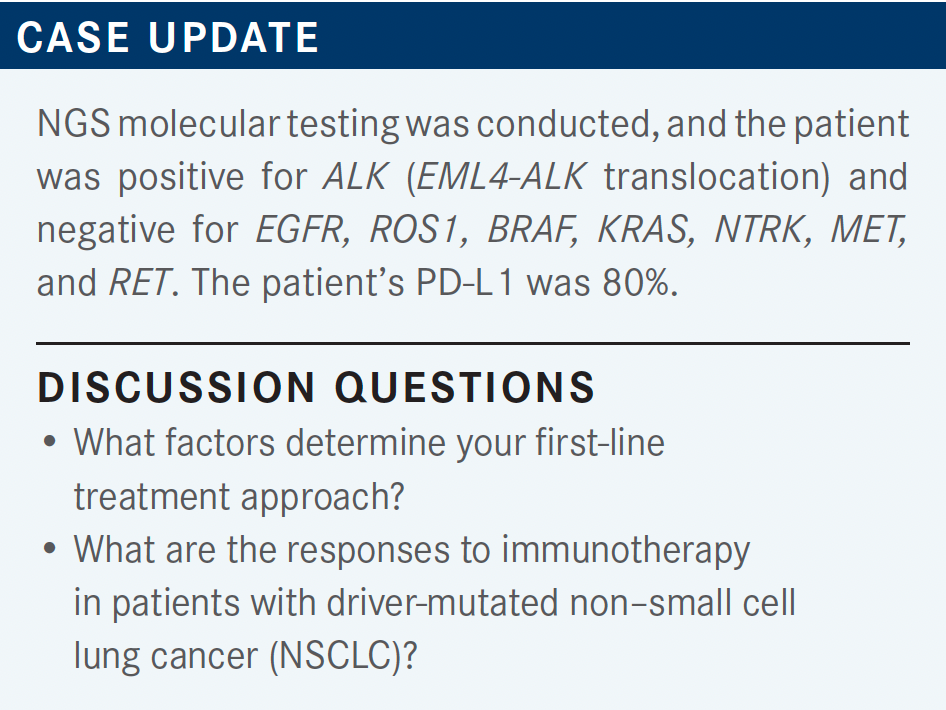
SOCINSKI: In the NCCN [National Comprehensive Cancer Network] recommendations, KRAS was added to the other usual suspects [described], and the recommendation is [optimally] for a broad molecular, panel-based, NGS approach.1 If there is insufficient tissue, the NCCN feels that [calls] for either repeat biopsy or plasma testing. [However,] it seems that many of us are doing concurrent tissue and blood.
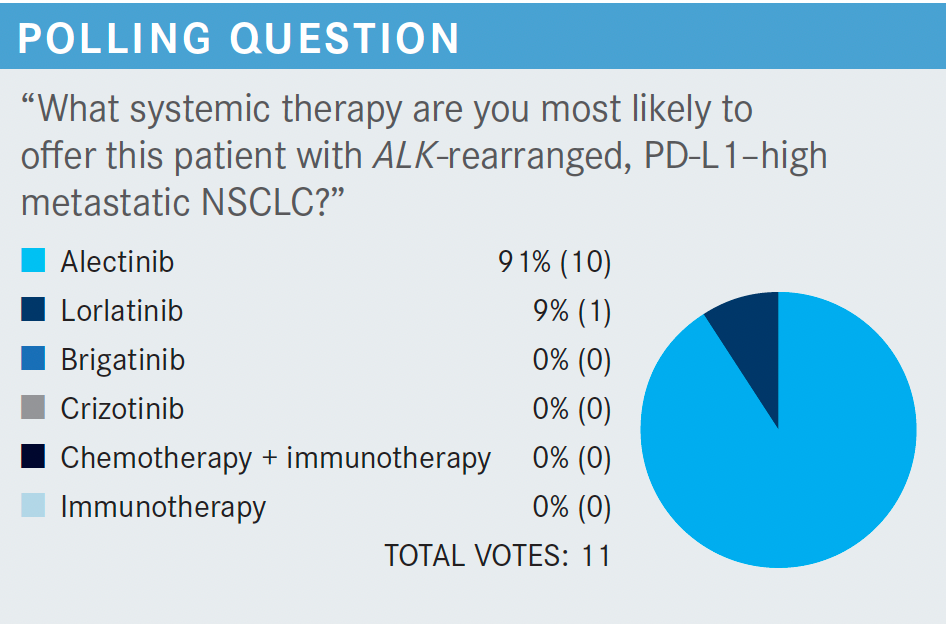
SOCINSKI: So why is everyone using alectinib [Alecensa]? Obviously in this gentleman, we found out about his ALK rearrangement prior to first-line treatment. [The NCCN] does offer up 3 treatments as category 1 options, but other recommended treatments include both ceritinib [Zykadia] and the runner-up category in certain circumstances is crizotinib [Xalkori].2
If, for some reason, you felt that you had to start treatment [right away], which does occur in this population, the recommendation is to complete the planned systemic therapy, including maintenance. If it’s working, I would say [to continue to use it], but if it’s not working, then interrupt and follow the other options that we had in the previously untreated population. Again, I think the NCCN frontline decision-making recommendations here are important, too.
We should have changed molecular testing for all biomarkers before pulling the trigger on first-line immunotherapy [in a case like this]. We do know that PD-L1 expression may be elevated in those patients with a targeted driver, but certainly the meaning of that may not be the same as it is in other patients [who don’t have it].
Certainly, targeted therapy in the presence of an oncogenic driver should take precedence over immunotherapy, and even in those patients with PD-L1 expression, obviously the targeted therapy is much more effective, with much higher response rates, and that’s what patients should be treated with.
MAKONI: I like both alectinib and lorlatinib [Lorbrena]. Alectinib is more tolerable than lorlatinib, especially because symptoms of hyperendemic are a real problem with lorlatinib. I used it only on 1 patient and they complained of some fogginess in the brain.
[Patients] sometimes complain about issues with lorlatinib, with the confusion on and off, but with alectinib I did not see any of those things. And I think that alectinib is much better tolerated than lorlatinib.
RAEZ: I have used lorlatinib in several patients because we have a trial here. I think it’s a great drug and I think that the market share all has elected to use lorlatinib first. My other observation with lorlatinib is unless we can prove that the lorlatinib benefit is as great as the sequencing of other agents [that was proven for EGFR already]. I’m very concerned about the more resistant mutations [that can be found] when you use lorlatinib. It’s a very nasty tumor compared with the treatment-resistant mutations when you use alectinib, and that’s why I still use alectinib first.
SOCINSKI: So your points are the attractiveness of using sequential therapy, and that we at least have data with lorlatinib. I think the point you were making was the attractiveness of the data that lorlatinib has as a second-line drug in this study.
LI: I think lorlatinib is less tolerated compared with alectinib. With alectinib, we most commonly see some gastrointestinal toxicity, constipation, and a nausea type of toxicity. However, for lorlatinib, I think central nervous system toxicity is more common.
SOCINSKI: How do you monitor for disease progression [when the patient is] receiving front-line therapy? What I do is I usually see the patients 2 weeks after I start the drug, for kind of a toxicity check. At that point if they have symptoms, generally the symptoms are getting better, and we tend to assume that the patient’s responding.
I’ll check the CT at 6 months, and then at 3 months. Then if people are doing well, I’ll do a CT scan, hecogenin based, every 3 months or so, unless they have an issue of some sort, in which case I’ll do it earlier. Does anyone have any different opinions, or any different strategies?
CARTWRIGHT: I typically have my nurse practitioner see the patient within a week or 2, just to get a little different perspective. Sometimes patients are quite intimidated [by oncologists], and [with nurse practitioners] they tend to ask different questions.
I just think it’s nice for the patients to hear somebody else, [that is, maybe not somebody who is perceived as quite] so intimidating. Not that [we are] intimidating, but the nurse practitioners take an approach that’s a little different.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Non-small cell lung cancer, version 5.2021. Accessed December 10, 2021. https://bit.ly/3lSCENw
2. Shaw AT, Bauer TM, de Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018-2029. doi:10.1056/NEJMoa2027187

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More