Aggarwal Examines Nivolumab Plus Ipilimumab in First-Line NSCLC
The use of chemoimmunotherapy as treatment of patients with treatment-naive stage IIIB or IV nonsquamous non–small cell lung cancer was the topic of a Targeted Oncology Case Based Peer Perspective discussion led by Charu Aggarwal, MD, MPH.
Charu Aggarwal, MD, MPH

The use of chemoimmunotherapy as treatment of patients with treatment-naive stage IIIB or IV nonsquamous non–small cell lung cancer (NSCLC) was the topic of a Targeted Oncology Case Based Peer Perspective discussion led by Charu Aggarwal, MD, MPH, Leslye M. Heisler associate professor for Lung Cancer Excellence at Penn Medicine in Philadelphia, PA.
Targeted Oncology™: What’s the rationale for using a triplet chemoimmunotherapy regimen in untreated stage IIIB or IV nonsquamous NSCLC?
AGGARWAL: I would point to the KEYNOTE-189 [NCT02578680] clinical trial, which was a large randomized clinical trial evaluating the use of triplet chemoimmunotherapy in patients with stage IIIB or IV nonsquamous NSCLC.1 These patients had no activating EGFR mutation or ALK translocation, and patients were randomized in a 2:1 fashion to receive the triplet [carboplatin or cisplatin plus pemetrexed (Alimta) and pembrolizumab (Keytruda)] every 3 weeks for 4 cycles followed by pemetrexed plus pembrolizumab maintenance or just the chemotherapy for 4 cycles followed by pemetrexed plus placebo maintenance followed by pembrolizumab every 3 weeks upon progression.
At a median follow-up of close to 2 years, the median overall survival [OS] was 22 months for the triplet, near doubling compared with chemotherapy alone with a hazard ratio of 0.56, and there was a tail to the [Kaplan-Meier] curve.2 There are some patients that are maintaining that benefit even at the almost 30-month mark.
The OS by different PD-L1 expressions, even for patients that had less than 1%, [showed] benefit with a hazard ratio of 0.52, and the benefit was clearly for all subsets, including for PD-L1 categories from 1% to 49% or greater than 50%. The patients who had tumors with PD-L1 expression greater than 50% seemed to be just hitting their median OS. It’s almost not reached with a hazard ratio of 0.59, so quite significant benefit with the use of triplet, even for this population.
If you look at progression-free survival data, I think it’s not as impressive for the PD-L1 less than 1% population, but overall in the total population, median progression-free survival has a hazard ratio of 0.48, and then there was an improvement seen across the other PD-L1–positive subgroups, 1% to 49% as well as the greater than or equal to 50%.
For the best overall responses, the response rates are much higher in patients who received the triplet. There are even some complete responders with triplet with an overall response rate that’s partial responses of about 48% with the triplet.
How do the findings from the KEYNOTE-042 trial affect your decision for treatment?
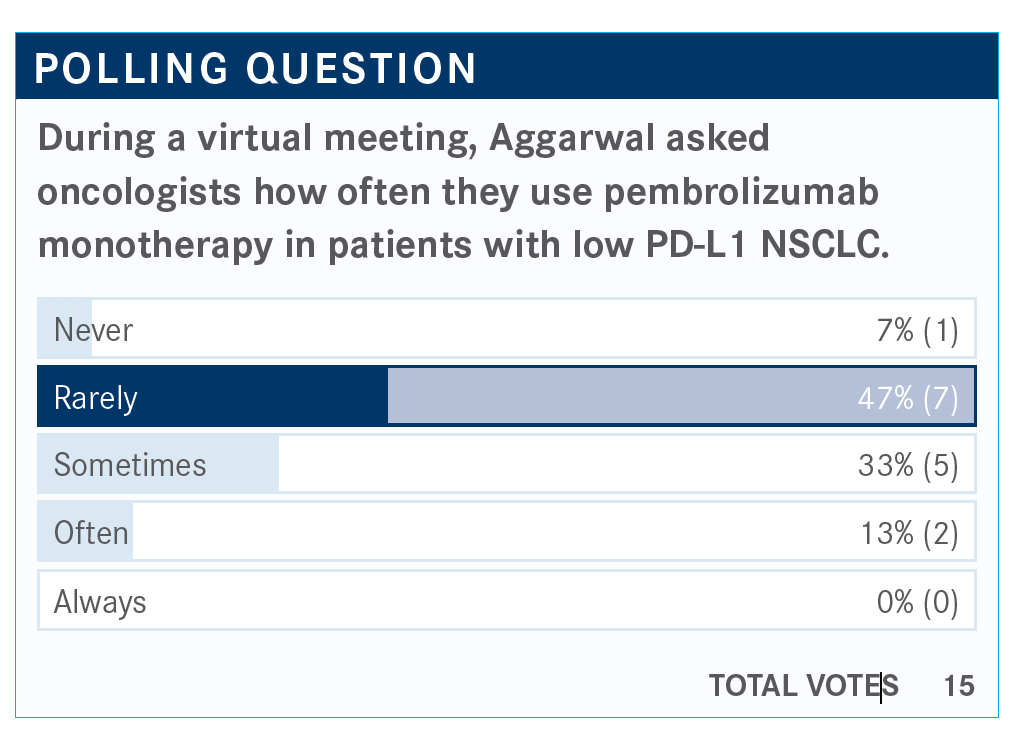
The data for using pembrolizumab in greater than 1% as monotherapy comes from KEYNOTE-042 [NCT02220894], which was presented in 2018 and 2019 at the American Society of Clinical Oncology Annual Meeting.3,4 This trial randomized patients with squamous and nonsquamous histology with metastatic NSCLC with a TPS [tumor proportion score] of greater than 1% to receive pembrolizumab monotherapy versus standard-of-care chemotherapy, which could be carboplatin plus paclitaxel for squamous disease or carboplatin and pemetrexed for nonsquamous disease. These patients had no history of untreated or unstable CNS [central nervous system] metastases. OS in patients with PD-L1 greater than 50%, greater than 20%, greater than 1% was the primary end point.
Patients who had a TPS of 1% to 19% [made up] about a third [of the cohort], and a majority of the patients were greater than 50%, so almost half of the patients had a high TPS 50% or greater score.
When we review the median progression-free survival [PFS], it doesn’t seem to be that significantly different from what we are seeing for chemotherapy alone with the exception of maybe the PD-L1 greater than 50%.
The median OS is 16.7 months with a hazard ratio of 0.81 compared with chemotherapy. I think the greatest benefit and the majority of the benefit was being derived from patients who had PD-L1 greater than 50% where we saw a hazard ratio that was close to 0.69 and then the PD-L1 greater than 20% were in the middle at 0.77 hazard ratio.
Looking at patients with PD-L1 to 49%, the hazard ratio was 0.92, so that demonstrates minimal advantage over chemotherapy alone. Some may argue that giving pembrolizumab monotherapy may be just as fine as giving chemotherapy in terms of efficacy for this population, but may avoid the toxicities, although I think if there is somebody who is able to receive triplet therapy should be able to receive it. On the other hand, I also hear the argument that there are some patients who just cannot receive chemotherapy, so this may be certainly a reasonable choice.
What are the implications of the IMpower150 [NCT02366143] trial?5
I think the study has been dubbed as the EGFR of immunotherapy study. Basically, this was the atezolizumab [Tecentriq] version of KEYNOTE-189 building upon the success of the initial carboplatin, paclitaxel, and bevacizumab [Avastin] backbone.
Patients with stage IV metastatic NSCLC that were treatment naïve were randomized into 3 arms. Arm A was atezolizumab plus carboplatin and paclitaxel. Arm B was the main interventional arm, which was the quadruplet, and arm C was the control arm [of] carboplatin, paclitaxel, and bevacizumab. Maintenance therapy was different. Patients taking the quadruplet [regimen] received atezolizumab and bevacizumab maintenance and they received atezolizumab maintenance alone for arm A, and bevacizumab maintenance alone for arm C. This trial allowed for up to 6 cycles of induction and patients were treated with atezolizumab as well as bevacizumab until disease progression.
In the quadruplet arm there was a significant improvement with a P value of .002, and there was an improvement, 19.2 months median OS for the quadruplet versus 14.7 months.
The OS for the intention-to-treat population, arm A versus arm C, shows that the addition of atezolizumab to carboplatin and paclitaxel did have somewhat of a benefit with a hazard ratio of 0.88. This was a trend. The efficacy boundary was not crossed, and this was not something that received approval, at least not from this trial.
The triplet for atezolizumab that led to its approval was IMpower130 [NCT02367781]. This evaluated patients with nonsquamous stage IV NSCLC randomized to receive atezolizumab plus carboplatin plus nab-paclitaxel [Abraxane] versus carboplatin plus nab-paclitaxel alone. Atezolizumab was the maintenance arm, and in this trial, patients could receive pemetrexed maintenance as a switch maintenance because they were starting with carboplatin and nab-paclitaxel. Patients were treated until progressive disease.
The hazard ratio was significant, 0.64, P value was significant, and the use of carboplatin plus nab-paclitaxel plus atezolizumab was published in Lancet Oncology. It led to an improvement in PFS as well as OS.6
What are the treatment options for patients with stage IV NSCLC with no EGFR or ALK alteration?
CheckMate 227 [NCT02477826] might apply.7 This was a complicated study, in patients with stage IV NSCLC who were treatment naïve and did not have an EGFR or ALK alteration. Patients were analyzed and stratified by 2 groups; greater than 1% PD-L1 or less than 1% PD-L1. The data that we saw most recently looked at the combination of nivolumab [Opdivo] plus ipilimumab [Yervoy] versus histology-specific chemotherapy in both PD-L1–positive and –negative subgroups.
Patients had PD-L1 greater than 1%. Median OS was 17.1 months for nivolumab plus ipilimumab, and chemotherapy was 14.9 months. This was a positive trial. This was one of the primary end points with a hazard ratio of 0.79.
Overall, if you include the PD-L1–negative patients, this trial was still positive with a hazard ratio of 0.73. The OS came in at about 17.1 months. This has a long follow-up with curves out to 42 months, and even with that long follow-up, we are seeing a median OS of about 17.1 months. For patients with PD-L1 less than 1%, median OS was about 17.2 months.
Regarding safety, the data for grade 3/4 diarrhea was about 1.7%, and then the other toxicities don’t seem that significant. Overall, 33% rate of all-event AEs does not seem that bothersome, at least at this point in time. I think some of this may be related to the slightly modified dose that was used.
Is there benefit to using a quadruplet regimen up front?
CheckMate 9LA [NCT03215706] is using the approach of quadruplet up front, giving a limited bolus of combination chemoimmunotherapy up front and then maintaining patients on nivolumab and ipilimumab later.8 Patients who were treatment naïve with NSCLC, no EGFR or ALK, were stratified by PD-L1, sex, and histology. Patients received nivolumab at 316 mg every 3 weeks, and ipilimumab was administered every 6 weeks. Chemotherapy was administered every 3 weeks for 2 cycles versus chemotherapy, which was every 3 weeks for 4 cycles. The primary end point was OS.
The median OS was 15.6 months versus 10.9 months for chemotherapy with a hazard ratio of 0.66. This has achieved approval based on the trial having met its primary end point. It does look like a positive trial, but I will point out that the chemotherapy arm, at 10.9 months, does seem to do quite as well as we would have expected it to do. Maybe 11 to 12 months may have been a better historical control.
Patients with PD-L1 less than 1% seemed to have a hazard ratio of 0.62. PD-L1 greater than 50% doesn’t seem to maintain that much of a differentiation in the long run, however. The curves look similar to each other, but there’s separation at 4 months or 6 months.
The disease control rate with the quadruplets was about 84% and 76% with chemotherapy. Overall response rate was about 38% with quadruplet versus 25% with chemotherapy. The duration of response was much longer with quadruplet at 11.3 months.
I think some of us have been concerned about using the quadruplet. About 358 patients on this trial were treated with the quadruplet and any treatment-related adverse event [TRAE], which was grade 3 or 4, was seen in about 47% of patients. However, serious TRAEs were in a quarter. Most common any-grade AEs were nausea, anemia, asthenia, and diarrhea.
The most common toxicities were skin, and there were some chemotherapy-related toxicities like anemia, neutropenia, alopecia, and thrombocytopenia. We didn’t see a lot of toxicity in terms of pneumonitis or colitis, but there was significant, as we would expect, endocrine primarily thyroid-related abnormalities that were seen, which we are all well versed with in managing.
Based on this trial, FDA approved nivolumab plus ipilimumab and chemotherapy for first-line treatment of metastatic NSCLC and again, this is not based on PD-L1 expression. Patients received 2 cycles of chemotherapy, and then they received nivolumab and ipilimumab maintenance after that.
References:
1. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi:10.1056/NEJMoa1801005
2. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from keynote- 189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505- 1517. doi:10.1200/JCO.19.03136
3. Lopes G, Wu Y-L, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(suppl 18):LBA4. doi:10.1200/ JCO.2018.36.18_suppl.LBA4 36
4. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. doi:10.1016/S0140-6736(18)32409-7
5. Socinski MA, Jotte RM, Cappuzzo F, et al; IMpower150 Study Group. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;14;378(24):2288-2301. doi:10.1056/NEJMoa1716948
6. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019 Jul;20(7):924- 937. doi:10.1016/S1470-2045(19)30167-6.
7. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med. 2018 May 31;378(22):2093- 2104. doi:10.1056/NEJMoa1801946.
8. Reck M, Ciuleanu TE, Dols MC, et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol. 2020;38(suppl 15):9501. doi:10.1200/JCO.2020.38.15_suppl.9501
