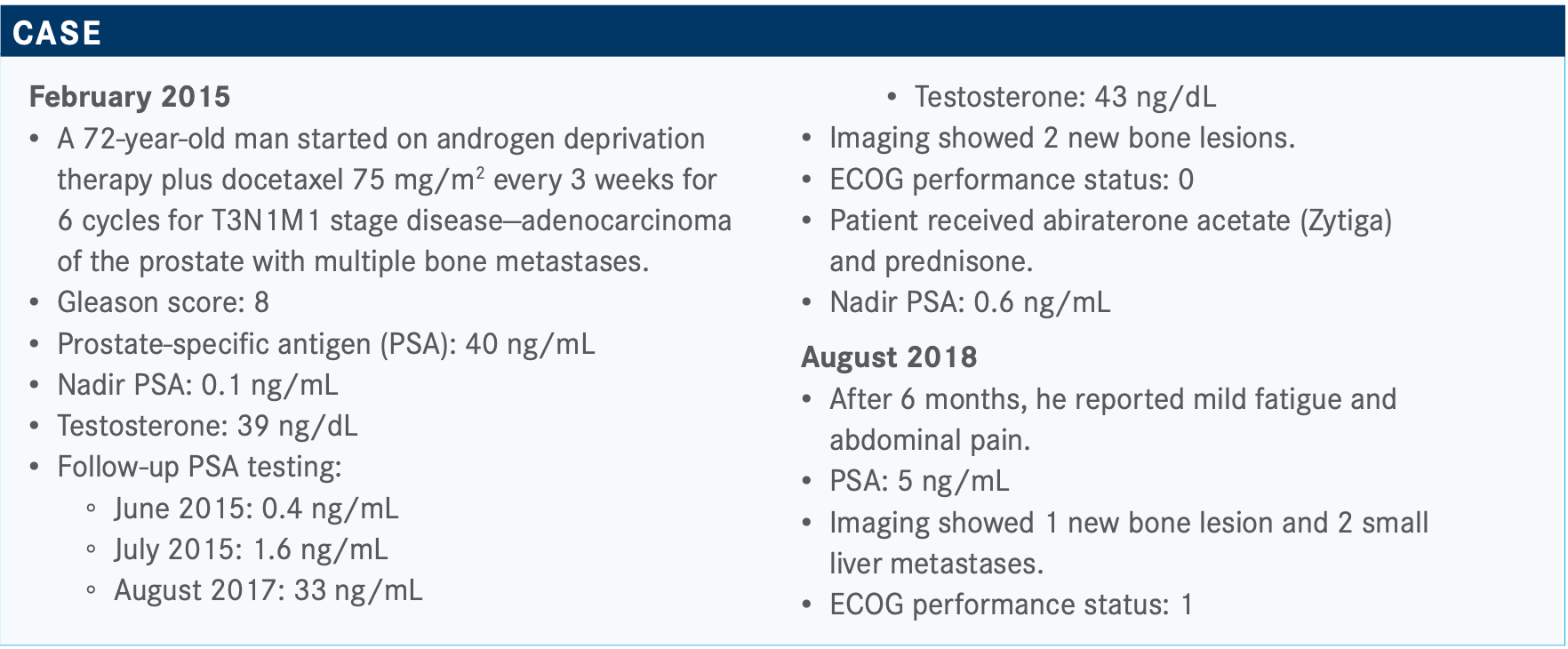
Vaishampayan Discusses Phase 3 Trial Results in mCRPC
During a Targeted Oncology Case-Based Peer Perspectives event, Ulka Nitin Vaishampayan, MBBS, FAB, discussed the case of a 72-year-old patients with metastatic castration-resistant prostate cancer.
Ulka Nitin Vaishampayan, MBBS, FAB

During a Targeted Oncology Case-Based Peer Perspectives event, Ulka Nitin Vaishampayan, MBBS, FAB, professor, Internal Medicine, director, Phase I Program Rogel Cancer Center, University of Michigan, discussed the case of a 72-year-old patients with metastatic castration-resistant prostate cancer.
Targeted OncologyTM: For a patient with metastatic castration-resistant prostate cancer (mCRPC), what are the treatment options after the disease progresses on abiraterone and prednisone?
VAISHAMPAYAN: Several second-line treatments are available but [it’s important to] conduct genetic profiling at this point [in the case], if not sooner. [Once that is done], chemotherapy, such as docetaxel or cabazitaxel [Jevtana], or second- line hormonal therapy can be considered.1
The CARD trial [NCT02485691], which was published in the New England Journal of Medicine in 2019, randomized a patient population to a second-generation hormonal therapy. If the patient first received enzalutamide [Xtandi], the patient then received abiraterone, or vice versa, and was compared with [those receiving] cabazitaxel and prednisone. The primary end point was radiographic progression-free survival [PFS].2
Most of these patients had advanced metastatic disease, and at least more than half of them were a Gleason score of 8 to 10 at diagnosis. The results showed that cabazitaxel significantly improved radiographic PFS. The median PFS with abiraterone or enzalutamide was 3.7 months compared with 8.0 months for those who received cabazitaxel. Overall survival [OS] also was statistically improved with cabazitaxel [13.6 months] compared with the hormonal therapy [11 months; HR, 0.64; 95% CI, 0.46-0.89; P =.008].
Regarding adverse events [AEs], patients given cabazitaxel experienced cytopenia, neutropenias, and diarrhea. About 20% of patients [who received cabazitaxel] had to stop because of AEs. Primary prophylaxis is typically recommended or should be considered with cabazitaxel.
Can you discuss treatment with PARP inhibitors for cases in which a BRCA mutation is present?
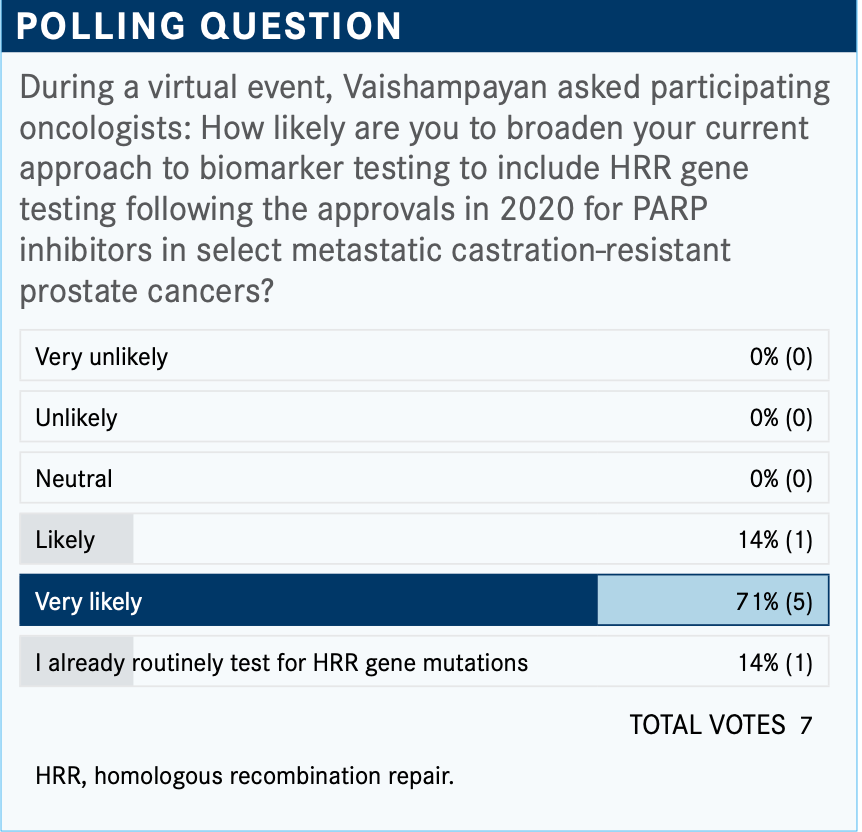
The phase 3 PROfound study [NCT02987543] led to the approval of olaparib [Lynparza] by the FDA. The trial included patients with either BRCA1, BRCA2, or ATM [mutations] in cohort A compared with patients in cohort B with mutations among 12 other genes involved in the [HRR] pathway, such as CDK12 and RAD51.3 The randomization was olaparib 300 mg twice a day or physician’s choice of treatment [enzalutamide 160 mg once daily] or abiraterone [1000 mg once daily plus prednisone 5 mg twice daily], and the primary end point was radiographic PFS.4
PFS was significantly improved with olaparib. In patients with BRCA1, BRCA2, or ATM alterations, median PFS more than doubled from 3.6 months in the control arm to 7.4 months [HR, 0.34; 95% CI, 0.25-0.47; P <.001].
Cohorts A and B together still show a benefit for olaparib [median PFS, 5.8 vs 3.5 months]. The hazard ratio here was 0.49, and the P value is statistically significant.
Secondary end points of OS also favored olaparib therapy [18.5 months] compared with physician’s choice [15.1 months]. Specifically in BRCA1, BRCA2, and ATM, there was a difference in cohort A. In cohort B, there was not a statistically significant difference.
[In] the overall population, the [OS] difference is seen [HR, 0.79; 95% CI, 0.61-1.03] but again not as big as what was seen in cohort A. Then you adjust for crossover, and even once you adjust for that, the difference is statistically significant favoring olaparib therapy [HR, 0.55; 95% CI, 0.29-1.06].
What toxicities are seen with olaparib?
Predominantly things to watch for with olaparib are cytopenias and gastrointestinal [GI] AEs, such as nausea, diarrhea, and constipation. There is a small risk of dizziness.
Are any other PARP inhibitors available in this setting?
The FDA approved olaparib for adult patients with deleterious or suspected deleterious germline or somatic HRR gene–mutated mCRPC.
Rucaparib [Rubraca] received an accelerated approval by the FDA for patients with BRCA-mutated mCRPC. The approval was based on a phase 2 trial [for which] patients were eligible if they had disease progression on both chemotherapy and at least 1 androgen receptor–directed therapy [TRITON2; NCT02952534].5
[Investigators screened] 1700-plus patients, 209 were enrolled, and 115 were evaluated for efficacy and safety. Most patients had a BRCA2 mutation (88.7%). The objective response rate was fairly remarkable at 43.5%, and the PSA response rate was 54.8% in the BRCA1/2 population, which was the dominate population within this group.6
Safety of rucaparib is similar to that of olaparib in terms of cytopenias and GI AEs.
What is the current understanding of the biology of mCRPC?
It is complex with notable inter- and intratumoral heterogeneity. Therapeutic sequencing is becoming a bigger and bigger issue. We have a number of agents that have been FDA approved back to back. However, we haven’t had time or adequate studies to sort out the exact sequence that may be beneficial. One thing is clear from the CARD trial—back-to-back abiraterone followed by enzalutamide or vice versa is not a good idea because of the outcome overall as compared with switching to chemotherapy. The chemotherapy arm did much better in terms of both PFS and OS and HRR. [Oncologists] can try to find a genomic target that they can address. For most patients, sequencing decisions are a big deal in prostate cancer, which can be determined based on the location of the disease, comorbidities, symptoms, and quality of life.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Prostate Cancer, version 3.2020. November 17, 2020. Accessed January 3, 2021. https://bit.ly/38bQ8gW
2.de Wit R, de Bono J, Sternberg CN, et al; CARD Investigators. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506-2518. doi:10.1056/NEJMoa1911206
3. FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. FDA. Updated May 20, 2020. Accessed January 3, 2021. https://bit.ly/3jIugw6
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant pros- tate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castra- tion-resistant prostate cancer. FDA. Updated May 15, 2020. Accessed January 3, 2021. https://bit.ly/2HGYMcy
6. Abida W, Patnaik A, Campbell D, et al; TRITON2 Investigators. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020;38(32):3763-3772. doi:10.1200/JCO.20.01035

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More