Use of Anthracycline or Nonanthracycline Therapy Is Considered in HER2+ Breast Cancer
During a Targeted Oncology Case-Based Peer Perspectives event, Sara M. Tolaney, MD, MPH, discussed therapies available for the treatment of a 42-year-old woman with HER2-positive breast cancer.
Sara M. Tolaney, MD, MPH

During a Targeted Oncology Case-Based Peer Perspective Roundtable, Sara M. Tolaney, MD, MPH, associate director, Susan F. Smith Center for Women’s Cancers, director, Clinical Trials, Breast Oncology, director, Breast Immunotherapy Clinical Research, senior physician, Dana-Farber Cancer Institute, and associate professor of Medicine, Harvard Medical School, discussed therapies available for the treatment of a 42-year0old women with HER2-positive breast cancer.
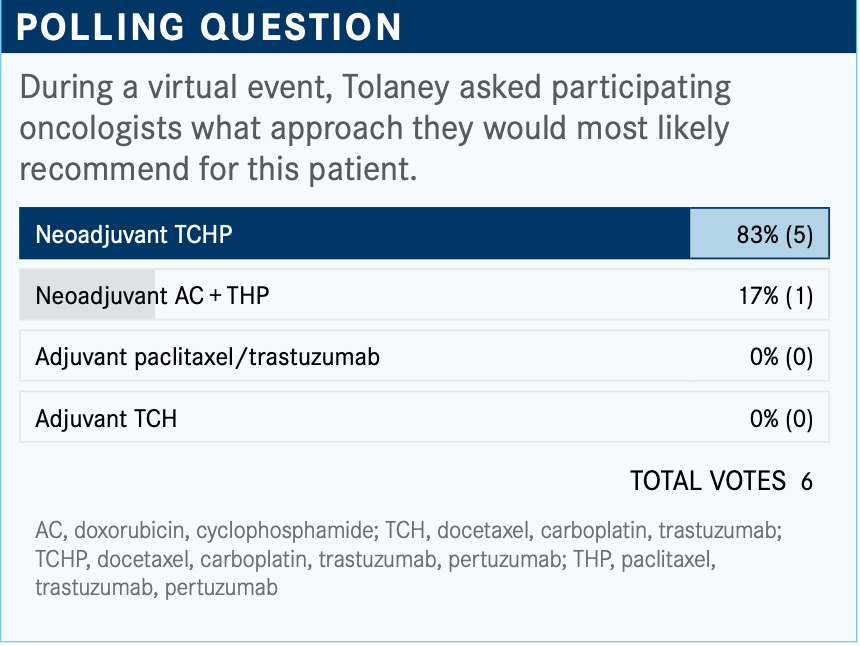
Targeted OncologyTM: What are your thoughts on the results of the poll?
TOLANEY: It looks like most people voted for TCHP [docetaxel, carboplatin, trastuzumab (Herceptin), pertuzumab (Perjeta)] and then 1 voted for AC [doxorubicin, cyclophosphamide] + THP [paclitaxel, trastuzumab, pertuzumab]. I think these are both reasonable choices. I think knowing that there is lymph node involvement is prompting people to use pertuzumab-based therapy. So, the question [is:] do you want to give anthracycline-based therapy versus nonanthracycline-based treatment? Given some newer data, I [tend] to now use nonanthracycline- based therapy for the vast majority of my patients since I have been using TCHP.
How would you assess this patient’s risk, and what factors should you keep in mind when assessing risk?
This was a fairly sizable tumor with nodal involvement [that was] high grade, HER2 positive, ER negative...These are all high-risk features. And so, certainly prior to the advent of trastuzumab [Herceptin], this patient would have a high risk of recurrence. The issue about ER will often come up as to whether or not it is truly a prognostic indicator in HER2-positive disease, where so much of the benefit from therapy is really driven by HER2.
If you look at long-term outcomes from HER2-positive patients in the adjuvant setting, and you were to compare the ER-positive versus ER-negative patients, you can see that their long-term outcomes are not that dissimilar. ER-positive, HER2-positive patients tend to do slightly better. They have a different time frame to recurrence, though. You’ll see that ER-positive, HER2- positive cancers tend to have recurrences a bit later than the ER-negative, HER2-positive cancers. But in the long run, when you follow them out 8 to 10 years, the difference is quite small between their long-term outcomes. But we do know that in a preoperative setting that the ER-positive, HER2-positive patients do have a lower likelihood of achieving pCR [pathologic complete response] to preoperative therapy, even though...their long-term outcomes are not that dissimilar.
Would you recommend neoadjuvant systemic therapy for such patients?
I find it hard to know what to do for that patient because if they ended up having, [for example], 1.2-cm HER2-positive cancer that was node negative, you take them straight to surgery and they could get away with just TH [paclitaxel, trastuzumab]. We know that their long-term outcomes will be good with TH [in the] stage I setting, with 98% recurrence-free interval out to now almost 7 years.1
But the problem that I have is I don’t know their nodal status, and I don’t really know how much cancer they have. So, I’m always a little worried that I’m either under- or over- treating them, depending on what you end up finding out later. Because of that fear, I have tended to take most of my patients [with masses] under 2 cm to surgery first, so that I know what [they are dealing with, in order to make] that final treatment decision.
It does put you at risk potentially for finding [a patient with] stage II disease, which is problematic, because I would have wanted to give them preoperative therapy. Then I could potentially give them postoperative T-DM1 [ado-trastuzumab emtansine; Kadcyla] if they had residual disease. But on the flip side, I worry that if the patient who I think has stage I disease ends up having stage II disease and has nodal involvement, then I’ve undertreated them. And so, I find it a little tricky to know how much to give them if [their tumor is] small. I think it’s a balance, because you don’t want to overtreat, you don’t want to undertreat, particularly when you are at this high 1s—1.8, 1.9 cm; that is where I struggle a bit.
There are a lot of studies ongoing that I think could...make this a little more comfortable. There is a preoperative trial through the [National Cancer Institute], the CompassHER2- pCR study [NCT04266249], which is looking at preoperative THP. And if someone achieves pCR, they go on to [receive] HP [trastuzumab, pertuzumab]. In my mind, if that study pans out and shows that we could potentially get away with THP in stage 2 patients, for example, then I would not feel so bad about giving my [patient with a] 1.8-cm mass THP up front because it was probably an effective amount of therapy; maybe they didn’t need the pertuzumab. We know from the APHINITY study [NCT01358877], for example, [that] there is no benefit from pertuzumab in our node- negative patients.
But at least I didn’t give them a ton of chemotherapy that they didn’t need; TCHP, in my mind, is difficult; with carboplatin, pertuzumab, they have a chance of diarrhea, so that’s not easy. Whereas weekly THP is easy for most patients; it’s not so bad. And so, we need to find a middle ground and we’re just not quite there yet. It sort of seems [as though] we’re picking between extremes of TH and then TCHP. And I think that’s part of what’s driving some of this problem.
For [a patient such as] this, it would be clear to do preoperative treatment.
Would you consider using anthracyclines in a patient such as this in the preoperative setting?
[You may] remember BCIRG 006 [NCT00021255], which compared TCH [docetaxel, carboplatin, trastuzumab] with ACT [AC followed by docetaxel] or AC-TH, showed that the anthracycline and nonanthracycline arms had fairly similar disease-free survival [DFS]. This was prior to pertuzumab becoming a standard, and it was also at a time when the study wasn’t powered to technically compare AC-TH to TCH, but we knew the DFS looked similar regardless of nodal status.2
But a more modern era trial is the TRAIN-2 trial [NCT01996267]. In this case, it was a different because they used paclitaxel with carboplatin and HP, not the traditional docetaxel and carboplatin. The comparator arm is also a little different than I typically would use because it’s FEC [5-fluorouracil, epirubicin, and cyclo- phosphamide] with HP followed by the taxane with HP.
What we showed was that the pCR was the same with either arm—anthracycline versus nonanthracycline. Now we have follow-up to almost 50 months,3 and the event-free survival is also the same. So, [there was] no statistically significant difference in event-free or overall survival.
There is also a subgroup analysis that showed that even if you looked at the high-risk, node-positive patients in this study, there was still no difference in event-free survival between the 2 arms. And [analysis showed] less cardiac toxicity, numerically less leukemia, and less febrile neutropenia with a nonanthracycline-based therapy.
Since these data have emerged, it has made me feel much more comfortable using nonanthracycline therapy because we’re seeing that, even with long-term follow-up in a modern era of pertuzumab-based therapy, there is no difference in long-term outcomes between anthracycline and nonanthracycline therapy. In my mind, there isn’t much rationale to consider anthracycline- based treatment for HER2-positive patients.
Do you agree with this approach?
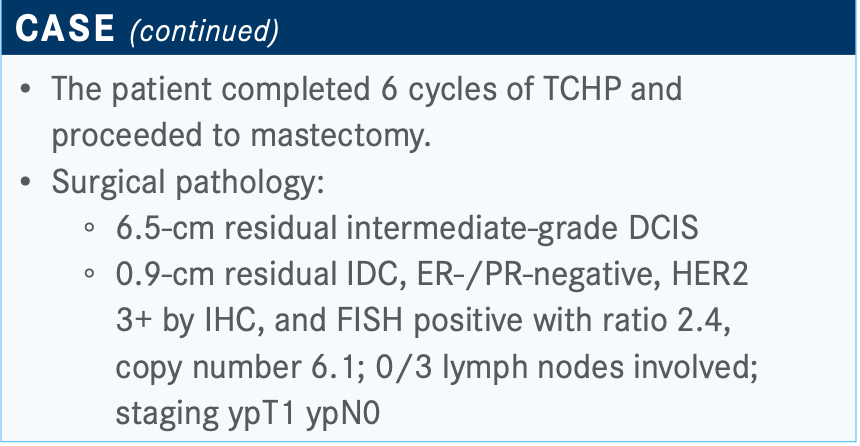
If you look at the preoperative trials, generally pCR rates are in the 60% range from TCHP. So, it is a highly effective preoperative therapy. We do know that those patients who achieve pCR have better long-term outcomes than those who have residual disease. And so, trying to maximize chances to get pCR makes sense for patients, and generally that is my approach, as well.
Obviously, there is interest in trying to develop less-toxic approaches. I fully admit that we’re seeing more toxicity with anthracycline-based therapy. I think we all are seeing that HER2- directed therapies are improving. With time we’re seeing better biologic treatments. There is a potential that we could get rid of some of the chemotherapy. Do we need to give everyone TCHP, I think, is the question. I think there are some patients who probably don’t need all this chemotherapy and probably would be fine with single-agent taxane and dual HER2-directed therapy. The problem is, how do we identify who those patients are? And who really needs the full TCHP?
I think [it’s being thought of now as], “Well, maybe we can use the achievement of pCR to help us figure that out.” Could we, for example, as COMPASS is doing, take THP and give it a test run and see if it works? Does it give a pCR? If it does well, we did good. If it doesn’t, then we need to escalate therapy, then we can give those patients who didn’t get the pCR more treatment. Could they get AC, followed by T-DM1, for example? They would have completed AC-THP and T-DM1 as if they had received AC-THP preoperatively and then T-DM1 out back.
I think this is what we’re trying to learn, how can we tailor therapy. We’re not quite there yet. Outside of clinical trials, I try to follow more textbook-like approaches, rather than these deescalation approaches, which I think should be reserved for trials at this point in time. I generally recommend TCHP.
What are some of these escalation/ deescalation strategies?
There are other trials that are ongoing in this space, and there are lots of deescalation approaches, [which try] to give less therapy. One deescalation approach could be to use T-DM1.
The KRISTINE trial [NCT02131064] had taken patients and randomized them to get TCHP or to get T-DM1 and pertuzumab. Technically the T-DM1 [arm], from a preoperative standpoint, had a higher pCR rate.4 But what was interesting to me is if you followed all the patients out for invasive disease-free survival [iDFS] after surgery, it was the same in both arms. So iDFS, if you follow people postop was exactly the same with T-DM1 + pertuzumab and TCHP.
The problem was in the preoperative setting. Some of the T-DM1 + pertuzumab patients had local regional progression, and those tended to be patients who had heterogeneous HER2 expression. If you think about it, these are patients who you’re trying to get to pCR by just HER2-directed therapy. So, if they have any HER2 heterogeneity, it’s not going to work perfectly right. If you have a HER2-positive 2+ patient who is borderline FISH-amplified, T-DM1 probably isn’t going to be a home run for this patient.
I think that’s another issue that’s going to come up as we try these deescalation approaches. Maybe it does need to be [an individual] who is strongly HER2-positive, 3+, to get away with an ADC [antibody-drug conjugate] such as T-DM1, which in my opinion, is much better tolerated than something [such as] TCHP and could be a wave of the future to try to deescalate therapy.
But on the flip side, I think we’re also trying to escalate treatment for the individuals who need it. There are trials ongoing trying to do more. One study, for example, in the high-risk population would be to potentially add immunotherapy to chemotherapy and HER2-directed therapy. There is a Roche study [NCT03135171] that’s adding a tocilizumab [Actemra] to AC-THP in the preoperative setting. And there are some investigator-initiated trials also adding pembrolizumab to THP preoperatively.
There are some studies that are trying to replace trastuzumab with margetuximab [Margenza] in the preoperative setting, trying to escalate therapy. There is work on both sides of the spectrum, trying to get the right amount of treatment to the right patient. I think as we learn more, hopefully, we’ll be able to do that. It’s going to be interesting to see if we’ll ever have biomarkers that will help us or if it will need to be something else.
There are some data that suggest early [PET scan] response could be a way to judge if you’re giving an appropriately deescalated therapy. That could be a way to have early markers of response to know if we can get away with a little bit less.
What’s your approach to a suboptimal response pathologically after neoadjuvant chemotherapy?
This patient received TCHP and had residual disease. Generally speaking, I tend to give 14 cycles of T-DM1, as per the KATHERINE trial [NCT01772472], because we know that that will reduce chances of recurrence by about half.5 But if they achieve pCR, then I tend to complete HP-based therapy outback. I think the question that will arise is, do you give [a patient] neratinib [Nerlynx] after completion of adjuvant HER2- directed therapy?
We have data from the ExteNET trial [NCT00878709], for example, that showed that giving a year of neratinib after completion of trastuzumab did have a significant improvement in iDFS.6 I think my challenge is that that study was done at a time when we were not giving pertuzumab. We were not giving postrehab T-DM1. And so, we don’t know what the benefit of neratinib would be in a modern-era patient.
That being said, the big benefit in ExteNET was in the hormone receptor–positive, HER2-positive patients, where, if you looked at the subgroup in that trial that had residual disease that was ER-positive and HER2-positive, there was a 7% absolute difference in iDFS and a trend toward survival benefit. And so, I have taken the stance, even though it’s completely not data driven, if [a patient] has ER-positive, HER2-positive disease and has residual disease after preoperative therapy, then goes on to complete their T-DM1 afterward, I offer neratinib.
What about the risk of diarrhea associated with neratinib?
[There are] some newer antidiarrheal approaches. The one that I tend to like best is the dose-escalation strategy, where you can start at just 2 pills a day and then escalate up a pill a week. [In my opinion,] just purine loperamide works well because patients tachyphylaxed to the diarrhea. If you start low and go up to full dose, I’ve noticed that they tolerate that well.
In fact, in the CONTROL trial [NCT02400476], which looked at various antidiarrheal regimens with neratinib, that was the best- tolerated approach with the lowest rate of discontinuation.7 The other approaches include budesonide with loperamide—so that’s the oral steroid that’s not absorbable or using colestipol, which is a bile acid sequestering [agent], and that works well with purine loperamide I find.
Any of those strategies—colestipol/loperamide, budesonide/ loperamide, or dose-escalation neratinib—have been much better than the days of using a purine loperamide, which doesn’t work as [we have seen] with the 40% rate of grade 3/4 diarrheas reported in ExteNET.6
If this patient has pCR, would you still do trastuzumab and pertuzumab because of the nodal status?
We have no answer because no one has done that study to see if you could deescalate the pertuzumab in a pCR patient. But there was a large meta-analysis, where they looked at patients who had a pCR and they looked at their risk of recurrence, and they found that baseline nodal status and tumor size were still prognostic. They were still independent prognostic indicators, even in a pCR patient. Those patients with upfront nodal involvement still have a higher rate of recurrence than the upfront node-negative patient with pCR; their baseline risk is still prognostic.
In this patient who does have high-risk [disease], we know that pertuzumab-based therapy for a year, based on APHINITY, did [lead to] risk reduction.
What is next in terms of adjuvant therapies?
There are studies that are ongoing trying to see if we can do even better than KATHERINE. Certainly, KATHERINE was very impressive with a 3-year iDFS of 88% with a 50% risk reduction.5 But there still was no reduction in CNS [central nervous system] recurrences in KATHERINE. And if you look at the node-positive subgroup of KATHERINE, their iDFS was about 83% at 3 years. So, in my mind, there’s still room for improvement in the high- risk, node-positive patients.
There are studies ongoing. One is called the CompassHER2-RD study [NCT04457596], which is looking at taking patients with residual disease and randomizing them to get T-DM1 or T-DM1 plus tucatinib [Tukysa] with the idea that there are some data to suggest tucatinib, the oral TKI [tyrosine kinase inhibitor] [works] synergistically with T-DM1. Could it enhance activity and potentially even prevent CNS recurrences, knowing that tucatinib penetrates CNS?
That study will take patients with residual disease, and, just [as in] KATHERINE, randomize them to T-DM1 with or without tucatinib. And then there’s a study opening through NRG [Oncology], looking at trastuzumab deruxtecan [Enhertu] in patients with residual disease [DESTINY-Breast05; NCT04622319]. It’s randomizing the residual disease patient to trastuzumab deruxtecan or T-DM1, again trying to escalate therapy for that high-risk patient.
I think we’ll see more with time about whether we can do even better for the high-risk patients, but for now, outside of a trial, T-DM1 would be the standard.
What adjustments have you made in treatment as a result of the coronavirus disease 2019 (COVID-19) pandemic?
One thing our group has started thinking about is using subcutaneous HP [Phesgo]. There are now data that compared the IV [intravenous] and subcutaneous formulations in the preoperative setting [from] the FeDeriCa study [NCT03493854].
The pCR rates were the same [59.5% vs 59.7%].8 They looked at pharmacokinetics between the 2, and they were also the same. [In terms of] adverse events, there weren’t any significant differences. There was a little more skin reaction at the site of the injection with the subcutaneous [formulation], but outside of that no real differences in toxicity profiles.
The other thing that I think is interesting, and I think this is a pretty cool study, is a preference study called PHranceSCa [NCT03674112], where they took people who received IV HP and then switched them to subcutaneous or did the reverse. They asked [the patients what they preferred], because every patient had been exposed to either formulation, and [more than] 80% of people preferred subcutaneous when they had received both.9
Obviously, the chair time and infusion are a lot less if you just give a 10-minute push of both drugs, rather than having to give 2 IV drugs 30 minutes each. Our group has started switching people over. It turns out when we compared the cost of subcutaneous with IV, subcutaneous was [less expensive]. I think Roche was clever in the way they priced it to try to encourage utilization. I think there are even some more advantages to chair time in the infusion center by using subcutaneous.
Our new practice has been that when we start, for example, TCHP, we just give everyone the subcutaneous formulation instead of IV. And then I’ve started offering my other patients who are in maintenance HP in the adjuvant setting after their pCR, for example, the subcutaneous version because they can be in and out of infusion much [more quickly].
We usually use the thigh [for the subcutaneous infusion], and you’re supposed to rotate location. It’s 1 shot, they’re both drugs are mixed into 1. That’s why it’s nice. The problem is, it is quite a bit of volume. And so, it’s, [for instance], 10 cc of fluid getting pushed subcutaneously and it has to be a very slow push for 10 to 12 minutes. So, it’s not like a quick subcutaneous push. But patients have really liked it.
There is a study ongoing where they’re sending nurses to [patients’ homes] and administering the HP there. I think particularly during COVID-19 there’s a big push to figure out how to do home administration of drugs. And since so many patients are on maintenance afterward, it’s nice for them not to have to come into clinic so much. I think we’ll see more about the feasibility of home administration, but for now, this has to be done by a nurse in our infusion center; it’s not something that can be done by patients at this time.
I also use the subcutaneous trastuzumab, as well. For example, when I give TH, I’ve switched over to using just subcutaneous trastuzumab, because that’s also quicker, particularly when you’re on just the trastuzumab maintenance. After the first 12 weeks, that’s in and out of infusion very fast. I haven’t had any insurance pushback, which is quite interesting, when using the subcutaneous trastuzumab formulation. It’s gone through just fine.
REFERENCES
1. Tolaney SM, Guo H, Pernas S, et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2–positive breast cancer. J Clin Oncol. 2019;37(22):1868-1875. doi:10.1200/JCO.19.00066
2. Slamon D, Eiermann W, Robert N, et al; Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273-1283. doi:10.1056/NEJMoa0910383
3. Van der Voort A, van Ramshorst MS, van Werkhoven ED, et al. Three-year follow-up of neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2-blockade for HER2-positive breast cancer (TRAIN-2): a randomized phase III trial. J Clin Oncol. 2020;38(suppl 15):501. doi:10.1200/JCO.2020.38.15_suppl.501
4. Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2017;19(1):115-126. doi:10.1016/S1470-2045(17)30716-7
5. Von Minckwitz G, Huang CS, Mano MS, et al; KATHERINE Investigators. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628. doi:10.1056/NEJMoa1814017
6. Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688- 1700. doi:10.1016/S1470-2045(17)30717-9
7. Barcenas CH, Hurvitz SA, Di Palma JA, et al; CONTROL Study Investigators. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann Oncol. 2020;31(9):1223-1230. doi:10.1016/j.annonc.2020.05.012
8. Tan AR, Im SA, Mattar A, et al. Subcutaneous administration of the fixed-dose combination of trastuzumab and pertuzumab in combination with chemotherapy in HER2-positive early breast cancer: Primary analysis of the phase III, multicenter, randomized, open-label, two-arm FeDeriCa study. Cancer Res. 2020;80(suppl 4):PD4-7. doi:10.1158/1538-7445.SABCS19-PD4-07
9. O’Shaugnessy J, Sousa SP, Cruz J, et al. Patient (pt) preference and satisfaction with the subcutaneous fixed-dose combination of pertuzumab (P) and trastuzumab (H) in pts with HER2-positive early breast cancer (HER2+ eBC): interim analysis of the open- label, randomised cross-over PHranceSCa study. Ann Oncol. 2020;31(suppl 2):S42. doi:10.1016/j.annonc.2020.03.020

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More