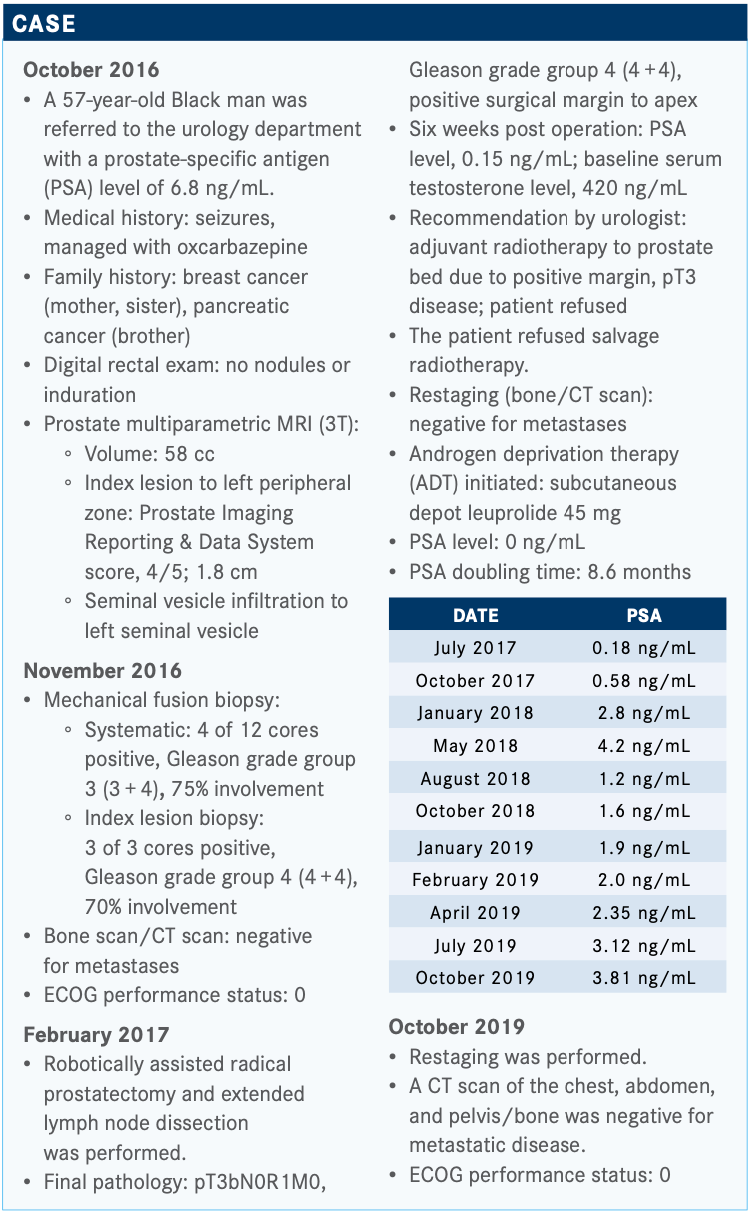
Physicians Have Multiple Choices of Treatment in the Nonmetastatic CRPC Setting
During a Targeted Oncology Case-Based Peer Perspectives Roundtable, Tanya Dorff, MD, reviews the available treatment options for nonmetastatic castration-resistant prostate cancer to select a treatment for 57-year-old patient.
Tanya Dorff, MD

During a Targeted Oncology Case-Based Peer Perspectives Roundtable, Tanya Dorff, MD, Genitourinary Cancers Program head, associate clinical professor, Department of Medical Oncology & Therapeutics Research, City of Hope, reviews the available treatment options for nonmetastatic castration-resistant prostate cancer (nmCRPC) to select a treatment for 57-year-old patient.
Targeted OncologyTM: What is your recommendation for the next line of therapy in this patient with nmCRPC?
DORFF: It’s hard if you don’t treat a lot of patients in this disease state. Now, apalutamide [Erleada] is also approved in metastatic castration-sensitive prostate cancer,1 so maybe more people are getting experience with it there.
When apalutamide was approved, I started using that exclusively. And then when darolutamide [Nubeqa] was approved, I began using that exclusively so that I could wrap my head around if there is a difference. People compare each agent with its placebo in the trial to look at differences and [to avoid] comparing across agents. People put a lot of stock in the fact that the darolutamide [chemical] structure should mean that it doesn’t cross the blood-brain barrier.
Clinically, I see the most neurologic adverse effects [AEs] with enzalutamide [Xtandi], like mild cognitive change or depression and anxiety, and I see less of it with apalutamide and less still with darolutamide. Things like fatigue are harder to get a feel for but [I’ll discuss] each agent versus its own placebo comparator to try to see if there’s a difference there.
But this patient had seizures, so with that medical history, it is probably safest to choose darolutamide.
What is the percentage of seizure rate for patients receiving darolutamide?
Across the board it’s low. It’s not like it’s a common event. However, someone with a seizure history is different from someone who was probably [treated] in the trial.
What if the patient had a BRCA or another mutation in the DNA repair damage (DRD) pathway? Would that change your treatment approach?
The jury is still out on that. If you look at the veliparib data from Maha Hussain, MD, a few years back, the patients with DRD mutations seem to do better with just abiraterone [Zytiga], regardless of veliparib.2
What do you think about the results of the poll?

The PSA doubling time is something I consider, and I calculate it for anyone I’m ordering these drugs for. I don’t know what my threshold would be [to choose darolutamide]. What if it’s 11 months versus 10 months? I think I would look at the Gleason [score] and comorbidities.
There might be the occasional patient with a doubling time lower than 10 months, and that person may be fine for a while and it might be the right thing to do to hold off therapy. But the patient’s anxiety definitely factors in.
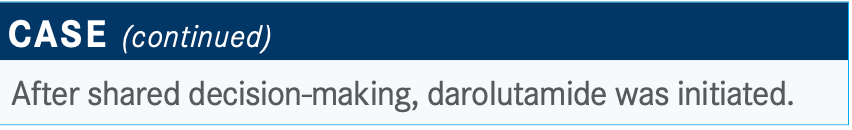
The NCCN [National Comprehensive Cancer Network] guidelines stratify [therapy selection] by PSA doubling time.3 It’s always important to check the testosterone level to make sure the patient is castration resistant and is not having testosterone breakthrough. First, slow doubling time observation is preferred, and for doubling time less than 10 months, apalutamide, darolutamide, and enzalutamide all have category 1 recommendations.
What trial supports the use of apalutamide in this patient?
The SPARTAN trial [NCT01946204] was with 1207 patients with nmCRPC. They did not exclude patients for small pelvic lymph nodes [<2 cm], but doubling time had to be less than or equal to 10 months. They were randomized to apalutamide 240 mg daily or placebo, [both with] ongoing ADT. The primary end point was metastasis-free survival [MFS].4
The baseline characteristics of patients treated on the SPARTAN trial were typical of what you would expect. Only 16% had pelvic lymph nodes but were still allowed to enroll and the majority had had prior definitive local therapy.
The primary end point of MFS for apalutamide was very positive, 40.5 months versus 16.2 months [HR, 0.28; 95% CI, 0.23-0.35; P <.0001]. The overall survival has also [been reported], which was insignificant [HR, 0.78; 95% CI, 0.64-0.96; P =.0161].5 I felt it was a hurdle that couldn’t be met this early in the disease, to be honest, when people are going to get subsequent lines of therapy.
The AEs showed more hot flashes, weight loss, and falls and fatigue [occur] more with apalutamide than placebo. There was a little more hypertension, too.
For apalutamide, thyroid[-related events] and rash are special AEs that we have to look out for. But they occurred in less than 15% of patients, so they’re uncommon. Just be aware that you have to check the thyroid periodically on apalutamide.
Are there any data indicating the use of enzalutamide in this patient?
Enzalutamide [was examined in the] trial PROSPER [NCT02003924] in M0 CRPC, 1400 patients. Baseline PSA had to be 2 or higher, so that’s unique. Enzalutamide was given at the standard 160-mg dose versus placebo and the primary end point was MFS.6
For the baseline characteristics, it’s shocking to see how high some of the PSA values are in nonmetastatic disease [<6 months in 77% of each arm], but the randomization was balanced in the 2 arms.
For the primary end point of MFS, enzalutamide was clearly better than placebo, at 36.6 months versus 14.7 months [HR, 0.29; 95% CI, 0.24-0.35; P <.0001]. Overall survival has also [been read out] now. It was significant [for enzalutamide (HR, 0.73; 95% CI, 0.61-0.89; P =.001)].7
Looking at AEs...we saw syncope, fatigue, and hypertension. But overall...compared with the placebo, enzalutamide looked pretty well tolerated.
Can you review the data supporting the use of darolutamide?
The darolutamide trial was called ARAMIS [NCT02200614]. Patients with nmCRPC and a baseline PSA greater than or equal to 2 ng/mL [were included]. The PSA doubling time was 10 months or less. They were randomized to darolutamide at 600 mg twice a day or placebo and the primary end point was MFS.8
There were 1509 patients treated in this trial. The patients’ baseline characteristics looked similar and well-balanced across trial arms. They allowed the [presence of] pelvic lymph nodes.
The primary end point of MFS for darolutamide was significantly improved, at 40.4 months compared with 18.4 for placebo [HR,0.41;95%CI,0.34-0.50;P<.0001].Overall survival was also significantly improved [HR, 0.69; 95% CI, 0.53-0.86; P =.003].9
The AEs [with darolutamide] compared with placebo showed more fatigue, but falls were not more common, which I was surprised to see. I haven’t seen that in my personal experience, but maybe I just haven’t treated enough [patients with this drug]. This was similar to placebo, which is why people talk about it having a good [safety] profile.
Can you use the results from these 3 trials to compare the agents?
People like to “stack up” the results of SPARTAN, PROSPER, and ARAMIS because they are so similar. I’m not trying to make cross-trial comparisons, but they had similar primary end points, 2:1 randomization, and the eligibility criteria were similar.
We can’t say from these data that any one is superior. They all look great, and I think this speaks to the fact that earlier treatment with better agents in prostate cancer makes a big difference. To me, this fits into the story of the metastatic hormone-sensitive disease trials [with abiraterone] like LATITUDE [NCT01715285] and STAMPEDE [NCT00268476]. Just treating it more intensively and more effectively earlier is better.
Are there any drug-drug interactions to keep in mind when prescribing systemic therapy to these patients?
[That depends on] whether we believe there’s a difference with the blood-brain barrier and drug-drug interactions.
There are some drug-drug interaction differences between the agents and that is something that is worth checking before you put a patient on one or the other. I always just run it through to make sure whatever medications they’re taking will be OK. Anticoagulants are one of the ones to watch out for.
Alicia K. Morgans, MD, [and colleagues released a] publication this year that looked at some different cardiovascular drugs and how those might interact with our different hormone therapy agents.10
REFERENCES:
1. Erleada. Prescribing information. Janssen Pharmaceutical Cos; 2018. Accessed December 21, 2020. https://bit.ly/34wVO2O
2. Hussain M, Daignault-Newton S, Twardowski PW, et al. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. J Clin Oncol. 2018;36(10):991-999. doi:10.1200/JCO.2017.75.7310
3. NCCN. Clinical Practice Guidelines in Oncology. Prostate cancer, version 3.2020. Accessed December 22, 2020. https://bit.ly/34xiIXZ
4. Smith MR, Saad F, Chowdhury S, et al; SPARTAN Investigators. Apalutamide treat- ment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408- 1418. doi:10.1056/NEJMoa1715546
5. Small EJ, Saad F, Chowdhury S, et al. Final survival results from SPARTAN, a phase III study of apalutamide versus placebo in patients with nonmetastatic castration-resistant prostate cancer. J Clin Oncol. 2020;38(suppl 15):5516. doi:10.1200/JCO.2020.38.15_suppl.5516
6. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resis- tant prostate cancer. N Engl J Med. 2018;378(26):2465-2474. doi:10.1056/NEJMoa1800536
7. Sternberg CN, Fizazi K, Saad F, et al; PROSPER Investigators. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197-2206. doi:10.1056/NEJMoa2003892
8. Fizazi K, Shore N, Tammela TL, et al; ARAMIS Investigators. Darolutamide in nonmet- astatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246. doi:10.1056/NEJMoa1815671
9. Fizazi K, Shore N, Tammela TL; ARAMIS Investigators. Nonmetastatic, castra- tion-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040-1049. doi:10.1056/NEJMoa2001342
10.Morgans AK, Shore N, Cope D, et al. Androgen receptor inhibitor treatments: Cardiovascular adverse events and comorbidity considerations in patients with non-meta- static prostate cancer. Urol Oncol. 2021;39(1):52-62. doi:10.1016/j.urolonc.2020.08.003
