Toxicities From Tafasitamab Monotherapy Are Less Severe After Discontinuing Lenalidomide in R/R DLBCL
The NCCN guidelines options available for patients with relapsed/refractory DLBCL include gemcitabine/oxaliplatin with or without rituximab, polatuzumab vedotin with or without bendamustine and rituximab, or the combination of bendamustine and rituximab alone.
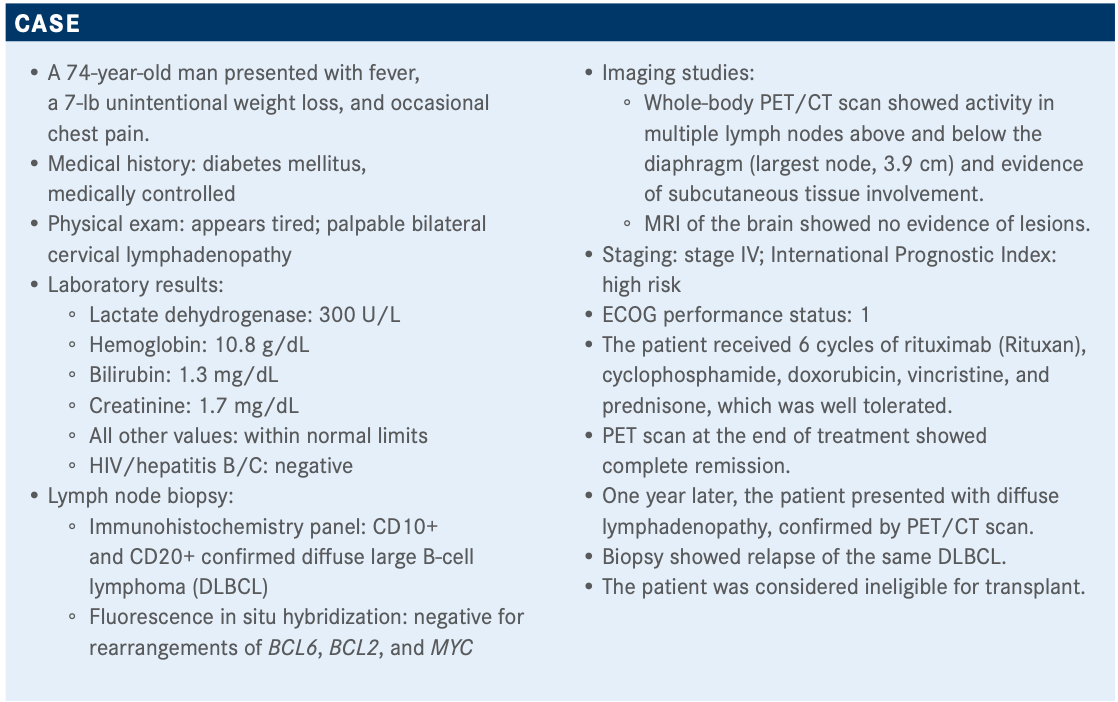
Targeted OncologyTM: What are the potential therapy options for this patient experiencing a relapse of his cancer?
BURKE: The NCCN [National Comprehensive Cancer Network] guidelines [for] options available for patients with relapsed/refractory DLBCL include gemcitabine/oxaliplatin with or without rituximab, polatuzumab vedotin [Polivy] with or without bendamustine and rituximab, or the combination of bendamustine and rituximab alone.1 Of course, bendamustine and rituximab have a label for 2 or more prior therapies, which this patient has not had; he has only had 1. But the study included patients in the second line, so it was a bit of an odd label based on the data.
Other chemotherapy regimens include CEPP [cyclophosphamide, etoposide, prednisone, and procarbazine], CEOP [cyclophosphamide, etoposide, vincristine, and prednisone], dose-adjusted EPOCH [etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin], and GDP [gemcitabine, dexamethasone, cisplatin], gemcitabine/vinorelbine, [all with or without] rituximab. Then the new March 2021 | Case-Based Roundtable Meetings Spotlight 17one is tafasitamab [Monjuvi] plus lenalidomide [Revlimid].
In certain circumstances, the NCCN listed brentuximab vedotin [Adcetris] for CD30+ disease. That does not have a label. There’s bendamustine with or without rituximab, ibrutinib [Imbruvica] for nongerminal center B-cell-like subtype. Also, selinexor [Xpovio] is listed as a third-line treatment. Chimeric antigen receptor [CAR] T-cell therapy probably has a separate category, but the American label on CAR T-cell therapy is for third line and beyond. My understanding is that you probably [would have] trouble getting this treatment paid for outside a clinical trial in the second-line setting right now.

What is the rationale for combining tafasitamab and lenalidomide, as this patient received, that led to approval by the FDA?
This patient received tafasitamab plus lenalidomide [after a relapse of his DLBCL]. On July 31, 2020, the FDA granted accelerated approval to tafasitamab plus lenalidomide for relapsed DLBCL.2
Tafasitamab, formerly known as MOR208, is an anti-CD19 monoclonal antibody. It has been modified on the Fc portion to increase its antibody-dependent cellular cytotoxicity and also antibody-dependent cellular phagocytosis. That is, it binds the macrophages to allow them to phagocytose the cancer cells as well, which causes some direct cell death. As a single agent, tafasitamab has modest activity, but where it really was thought to be more exciting was in improving the activity of lenalidomide. Lenalidomide can activate T and natural killer cells, and so the thought was that perhaps the increased antibody-dependent cellular phagocytosis and cellular cytotoxicity aspects of tafasitamab combined with lenalidomide would be synergistic. Lenalidomide has some activity in large cell lymphoma and it is proven in other lymphomas, but it was not approved in DLBCL.
Which trial looked at this combination, and what was the design of the study?
The hypothesis [for tafasitamab plus lenalidomide] was tested in a clinical trial called L-MIND [NCT02399085].3This was a phase 2 single-arm open-label study. Patients with relapsed/refractory DLBCL were eligible for the trial, but they had to have received 1 to 3 prior regimens and they had to not be eligible for stem cell transplant.
The trial excluded most patients with primary refractory disease. This was a relatively favorable population because they excluded some of the [patients with worse disease]. Patients who enrolled went on to receive the combination of tafasitamab and lenalidomide. Lenalidomide was dosed—a big dose—at 25 mg daily on days 1 through 21 of a 28-day cycle, 3 weeks on, 1 week off. For the first 3 cycles, tafasitamab was dosed weekly on days 1, 8, 15, and 22. There was also loading dose during week 1 on day 4, but that was just cycle 1. After the first 3 cycles the dosing schedule of tafasitamab was changed to days 1 and 15, so every other week. It was continued through 12 cycles and after those 12 cycles, lenalidomide was stopped and tafasitamab was continued as maintenance long-term treatment until [disease] progression. This is indefinite therapy, as we do with some other diseases, but we are not used to that with DLBCL. The primary end point of this study was objective response rate [ORR], and the secondary end point included progression-free survival [PFS] and overall survival [OS], and duration of response [DOR].
Eighty-one patients were enrolled on [L-MIND] and their median age was 72. The median prior lines of therapy was 2, but about half of patients have received only 1 prior line of therapy. The other half had received more than that. So as relapsed large cell lymphoma trials go, you can compare this with the others, but this was a relatively less heavily pretreated population. Eleven percent received a prior stem cell transplant, 44% were refractory to their last prior therapy, and although I [mentioned] that they excluded primary refractory disease, they actually included a few. Nineteen percent looks like kind of a complicated story, but they actually changed the trial design a little bit as the trial was going on.
What were the efficacy data seen in the L-MIND study that led to approval of the therapy?
The ORR was 60% [95% CI, 48%-71%], which is high in a relapsed/refractory DLBCL trial, and the complete response [CR] rate was 43% [95% CI, 32%-54%]. The median DOR was 22 months and the DOR, broken down by whether it was partial response [PR] or CR, [showed] patients who achieved a CR had much better duration of response than those who achieved only a PR. If a patient achieved a CR, the median duration was not even reached at 2 years, whereas if you had a PR, the median duration was 4 months.
Median PFS was 12 months [95% CI, 5.7%-not reached]. The median OS was not yet reached [95% CI, 18.3–not reached]. At 12 months, 74% of patients were still alive, at 18 months it was 64%, and the number of deaths between 12 and 18 months was pretty low.
What was the toxicity profile of this therapy that oncologists should be aware of?
The key toxicities [found on the L-MIND study] are neutropenia, anemia, and thrombocytopenia. Grade 3 neutropenia was 27% and grade 4 at 21%, so a total 48% of patients [were] getting grade 3 or 4 neutropenia. Any-grade febrile neutropenia was only at 12%, so it was not significant, but that’s one of the key hematologic toxicities to keep in mind.3
The [most common] nonhematologic toxicities were fatigue, diarrhea, cough, fever, edema, respiratory infection, and loss of appetite. Grade 3 and 4 rash sticks out at 9%, and most of the rest are pretty uncommon. Diarrhea was common at 32%.
Fifty-one percent [of patients on the trial] had serious adverse events [AEs], with 12% discontinuing combination therapy due to an AE. Nineteen of these serious AEs were thought to be related to treatment and 13% of these events led to death. Upon discontinuation of lenalidomide after cycle 12, or earlier if they had toxicities, the rate of these AEs went down during tafasitamab monotherapy.
Toxicities that occurred during the first 12 cycles of combination therapy and toxicities with tafasitamab monotherapy in 37 patients, who either made it to a year or they came off lenalidomide because of toxicities, [went down]. The number of patients with grade 4 neutropenia went down [to 19 with the combination therapy and 5 with the tafasitamab monotherapy] as [did the number of] patients with anemia, [34 patients grade 1-3 compared with 10 patients with grade 1 anemia, respectively]. Basically, everything went down. This indicates that most of these toxicities are due to lenalidomide, and when the patients can get through that first year again on tafasitamab monotherapy, the toxicities go down.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 4.2020. Accessed February 16, 2021. https://bit.ly/35H3YW0
2. FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. FDA. Updated August 3, 2020. Accessed February 16, 2021. https://bit.ly/34Emq2z
3. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More
Examining the Non-Hodgkin Lymphoma Treatment Paradigm
July 15th 2022In season 3, episode 6 of Targeted Talks, Yazan Samhouri, MD, discusses the exciting new agents for the treatment of non-Hodgkin lymphoma, the clinical trials that support their use, and hopes for the future of treatment.
Listen