REACH-2: Ruxolitinib Is Viable Option in Steroid-Refractory Acute GVHD
Experts decide whether ruxolitinib is a viable treatment option for steroid-refractory acute graft-versus-host-disease based on the case of a 48-year-old patient.
Miguel-Angel Perales, MD

Experts decide whether ruxolitinib (Jakafi) is a viable treatment option for steroid-refractory acute graft-versus-host-disease based on the case of a 48-year-old patient.
Targeted OncologyTM: Is this patient’s case the standard of care in this setting? What are the possible risk factors here?
PERALES: In general, the more ablative conditioning regimens have been associated with a higher risk of GVHD. For a younger patient with AML, the standard of care would be considered an ablative transplant based on the recent BMT CTN [Blood and Marrow Transplant Clinical Trials Network] 0901 study [NCT01339910], which compared an ablative transplant with reduced-intensity conditioning and showed a 3-fold increase in relapse in patients with AML who had a reduced-intensity transplant.1 That’s definitely one of the risk factors.
[Another risk factor is the] donor, a 50-year-old woman with 3 kids and also a [CMV-seropositive] donor. What we know from registry data is that the younger male donor is the preferred donor, so if you had a 25-year-old male and a 50-year-old female multiparous, it would be pretty clear-cut who you would go with. Maybe this was the only donor.
Some of the risk factors: The major one is human leukocyte antigen disparity, which was not the case in this patient; sex matching; donor parity; and donor age. Other potential factors are CMV status, ABO mismatch, and the cytokine gene polymorphisms. Those are probably minor factors.
In terms of stem cell graft factors, there is more GVHD with PBSC than with bone marrow. There is more GVHD with both of those sources compared with cord blood, as long as the cord blood is equally matched. One of the advantages of cord blood is that we can use cord blood as only a 4 of 6 match with similar results in terms of survival to a fully matched adult donor, but in that case the GVHD is at least equivalent to what we would see with a PBSC graft.
We know that a higher CD34 count is associated with higher T-cell dose and therefore also with more GVHD. Myeloablative conditioning is associated with higher acute GVHD.

What do you think of this patient’s updated case and these poll results? What stage and grade of GVHD does this patient have?
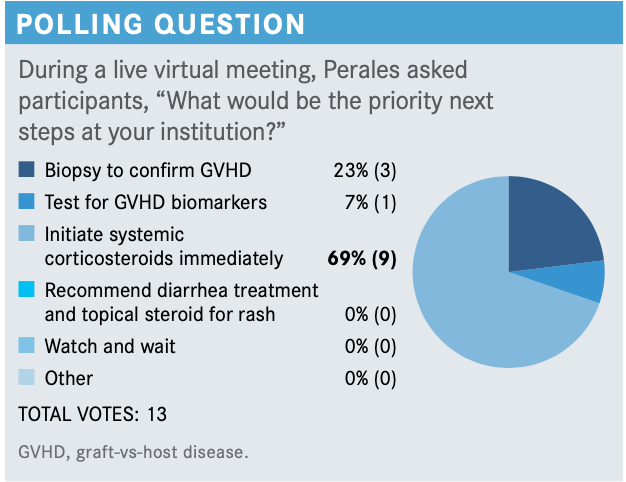
I think this sounds like a clear-cut case of GVHD with both skin and gut involvement. The use of biopsy is always useful to confirm a diagnosis, but in some cases, it’s nondiagnostic and is not an absolute requirement, so I agree that in this patient, starting steroids makes sense.
The skin involvement was 60%, which makes it a stage 3 skin [involvement], and then there was no indication of liver or upper gut involvement—and [I am] using the Mount Sinai Acute GVHD International Consortium [MAGIC algorithm], which incorporates both volume of stool or, in patients where you can’t measure the stool, particularly in the outpatient setting, the number of episodes per day.2 This patient had at least 4 episodes a day, so that would be a lower gut [involvement], lower gastrointestinal [GI] stage 1. The consortium is based on the most severe organ involvement, and so the stage 3 rash [and] stage 1 lower GI would make [this patient’s disease] grade II.
Can you discuss some other scoring systems for acute GVHD?
Another grading system that people are currently using is the Minnesota severity grading system.3 This basically takes the standard GVHD staging and grading system and divides it into 2 groups, standard risk and high risk. The distribution for standard risk is primarily skin involvement or skin and lower stages of gut, as in this patient, whereas high risk is pretty much anything that is a stage 3 nonskin, stage 4 skin, or multiple organ involvement.
The MacMillan, [et al], paper is useful on this Minnesota severity scoring system. The overall response rate [ORR] to steroids in the standard-risk patients was about 70% with almost 50% complete response [CR] rates; whereas, in the high-risk group, you cut that in half with just over 40% ORR and 27% CR rates with steroids at day 28.
The nonrelapse mortality at 6 months was 22% in the standard-risk population, and double that, 44%, with the high-risk population. This allows you to stratify your patients at time of diagnosis. The current standard of care is steroids, but there are several studies that are looking to improve on that by risk-stratifying patients up front, then randomizing patients in the high-risk group to additional therapy. The BMT CTN, for example, is doing a study right now with a₁-antitrypsin [AAT], where they randomize high-risk patients to steroids plus or minus AAT [NCT04167514].

How is this patient doing? What are the goals for treatment?
This was a good response, not a CR, but a very good response to the steroids.
The primary therapy for acute GVHD has several goals. One is to improve or stabilize the organ manifestations. Two is to limit the long-term treatment-related toxicities. Another is improvement in functional capacity or quality of life. Currently, the first-line therapy in this setting is treatment with corticosteroids, and the only drug approved for second-line therapy is ruxolitinib. The goal is to improve overall survival.
How do you taper steroids after achieving initial response?
I think the steroid taper is one of the hardest parts of managing GVHD. Most protocols will have a recommended schedule. The main goal is to try to get the patients off as quickly as possible, and what we sometimes run into is the patients who are doing well are not coming to clinic as often and get tapered slower because you’re not seeing them. I think the best approach is typically to give the patient a calendar of steroid tapering that they would follow and only deviate if they start to have more symptoms of flare. But in the absence of a calendar, I think what we often end up seeing is that it can vary from attending physician to attending physician and from patient to patient. We try to provide patients with a more formal approach to tapering the steroids, but the ultimate goal is to get them off as quickly as you can.

Are there any approaches that you take that you think are associated with better outcomes?
Early recognition and early intervention are important. I think good collaboration with your dermatologist and your gastroenterologist is helpful. [Also] recognizing differential diagnosis, drug rash typically. In terms of GI, ruling out infections: That often requires stool studies as well as a biopsy. The incidence [of GVHD]...can increase if you do a lot of mismatch transplants, it can decrease if you switch to posttransplant cyclophosphamide, or, in the case of our approach, we see much lower rates of acute GVHD.
We’re awaiting the results of 2 large national studies. The first study is known as BMT CTN 1301 or Progress II [NCT02345850], and that study randomized patients to tacrolimus/methotrexate, posttransplant cyclophosphamide or [CD34-selected PBSC] and may have an early readout before the end of the year, but too late for an American Society of Hematology presentation.
The other study is Progress III or BMT CTN 1703 [NCT03959241], which is in the reduced intensity setting comparing posttransplant cyclophosphamide and tacrolimus/mycophenolate mofetil versus tacrolimus/methotrexate in patients getting PBSC grafts. That study is ongoing, so that will probably have an 18- to 24-month readout. I think those are 2 of the large national phase 3 trials that may give us more information both in the ablative and reduced-intensity settings.
What are the different ways patients’ disease interacts with steroids?
The definitions that we typically use around refractoriness or resistance—that’s defined as progression within 3 to 5 days on the high-dose steroids, failure to improve within 5 to 7 days, or the incomplete response after more than 28 days4—you want to see a response by 1 week basically.
Dependence is defined as patients whom you can’t drop the dose for, who recur after tapering. It’s a patient who is stuck at 2 mg/kg or you drop to 1.5 mg/kg and then they flare again.
Intolerance is patients who have significant adverse effects and can’t tolerate the high-dose steroids.
How have the National Comprehensive Cancer Network (NCCN) guidelines changed in this setting?
The NCCN has recently issued guidelines in the field of transplant, and one of the first things they targeted was GVHD.5 What they’re giving is, I would say, more information as opposed to specific treatment guidelines, like they might do in the solid tumor settings where they give a treatment algorithm.
They list a number of drugs both for acute and chronic GVHD. There are 2 approved drugs: ruxolitinib, which is FDA approved in acute GVHD, and ibrutinib [Imbruvica], which is FDA approved in chronic GVHD. All the other drugs listed have been tried mostly in single-center studies and have some data, but in many cases, [the data haven’t] been reproduced or used in phase 3 trials. I see that in many cases, a clinical trial is the best treatment.
What are some of the data for the drugs in the NCCN guidelines like ruxolitinib?
The REACH-1 study [NCT02953678] was a multicenter phase 2 trial that Memorial Sloan Kettering Cancer Center participated in.6 It enrolled patients with steroid-refractory acute GVHD, 71 patients were included, and all these patients were treated with ruxolitinib initially at 5 mg twice a day with the option to escalate to 10 mg twice a day after 3 days. The primary end point was ORR at day 28. This study was positive in terms of [this end point] and led to approval by the FDA of ruxolitinib in this indication.7
The ORR was 55% with an almost 27% CR rate, and the best ORR during treatment was 73% with a CR rate of 56%.8 The median time to response was 7 days with a range from 6 to 49 days, and the median duration of response was up to 345 days.
Unfortunately, one of the main issues is that the mortality rate was still significant with almost half the patients dying. The nonrelapse mortality at 6 months was 44%.
These data, which I mentioned, led to approval in the United States on the basis of a single-arm study. In Europe they requested a phase 3 trial, which is the REACH2 study [NCT02913261]. This was in patients with steroid-refractory GVHD, so it had similar inclusion criteria. Patients were randomized to either ruxolitinib 10 mg twice a day or best available therapy [BAT]. The primary end point was again the day 28 ORR, and patients had the option to cross over from BAT to ruxolitinib if they progressed.
How did patients do on ruxolitinib in REACH-2?
These data were published in the New England Journal of Medicine by Robert Zeiser, MD.9 These results were quite consistent with what was seen in the phase 2 trial. The ORR at day 28 was 62%, with a 28% partial response and 34% CR rate, and that was significantly better than what was seen with BAT. This was a large study, just over 300 patients. The durable ORR at day 56 did decrease, but it was still significantly in favor of ruxolitinib.
On the basis of this, ruxolitinib is now also approved in Europe, and I think these data validate the United States experience, so I think many centers now consider ruxolitinib the standard of care for steroid-refractory GVHD. Because of potential myelosuppression, some patients are not candidates for ruxolitinib, and those are patients where a clinical trial can be considered.
Looking at the loss of response, there was a significant difference between patients on ruxolitinib and the control group. There were stable responses in patients who respond, so ruxolitinib was durable.
In the different organs—skin, upper GI, liver, lower GI—if we look at the skin as an example, the starting point was relatively similar between ruxolitinib and BAT, and there was more response in the skin in the ruxolitinib arm compared with the BAT arm at day 28. In the lower GI in particular, there was certainly more response in the ruxolitinib group.
The failure-free survival curve showed a significant difference between the 2 groups in favor of ruxolitinib.
What are some of the potential toxicities associated with ruxolitinib?
The ones that stand out are increased rates of thrombocytopenia with ruxolitinib, and also a slightly increased risk of CMV. There have been some reports of CMV with ruxolitinib, and that’s why this was looked at more carefully [in REACH-2]. There didn’t appear to be a marked difference. I would say the main difference we see in the clinical experience is the thrombocytopenia.
REFERENCES
1. Scott BL. Long-term follow up of BMT CTN 0901, a randomized phase 3 trial comparing myeloablative (MAC) to reduced intensity conditioning (RIC) prior to hematopoietic cell transplantation (HCT) for acute myeloid leukemia (AML) or myelo-dysplasia (MDS) (MAvRIC Trial). Biol Blood Marrow Transplant. 2020;26(3):S11. doi:10.1016/j.bbmt.2019.12.075
2. Harris AC, Young R, Devine S, et al. International, multicenter standardiza-tion of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22(1):4-10. doi:10.1016/j.bbmt.2015.09.001
3. MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761-767. doi:10.1016/j.bbmt.2015.01.001
4. Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018;53(11):1401-1415. doi:10.1038/s41409-018-0204-7
5. NCCN. Clinical Practice Guidelines in Oncology. Hematopoietic cell transplanta-tion, version 2.2020. Accessed February 15, 2021. https://bit.ly/3ocIjNV
6. Jagasia M, Zeiser R, Arbushites M, Delaite P, Gadbaw B, Bubnoff NV. Ruxolitinib for the treatment of patients with steroid-refractory GVHD: an introduction to the REACH trials. Immunotherapy. 2018;10(5).391-402. doi:10.2217/imt-2017-0156
7. FDA approves ruxolitinib for acute graft-versus-host disease. FDA. May 24, 2019. Accessed February 15, 2021. https://bit.ly/2HgBNoQ
8. Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739-174
9. doi:10.1182/blood.20200048239. Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for glucocorticoid-refrac-tory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800-1810. doi:10.1056/NEJMoa1917635

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More