Lipe Discusses Therapeutic Options for a Patient With Transplant-Ineligible Multiple Myeloma
"I think it’s important to know that this can change over time, what is defined as transplant eligibility, so getting the transplant center involved early can be helpful."
Brea C. Lipe, MD

Brea C. Lipe, MD, associate professor, Department of Medicine, Hematology/Oncology (SMD), clinical director, Multiple Myeloma Program, Wilmot Cancer Institute, University of Rochester Medical Center, discussed the case of a 72-year-old patient with multiple myeloma during a virtual Targeted Oncology Case-Based Roundtable event.
Targeted OncologyTM: What criteria do you use to define transplant eligibility?
LIPE: I think [one] thing to remember is that someone you see initially, who might be transplant ineligible, might be suffering from the disease itself, and their performance status can change. I think it’s important to know that this can change over time, what is defined as transplant eligibility, so getting the transplant center involved early can be helpful.
So is there an upper limit to age? I think, at our institution, there isn’t an upper limit. We’ve transplanted patients in their 80s. I didn’t know that this was necessarily the best idea, but some of them have done just fine. Performance status is key and organ function is important. If they have concomitant amyloidosis, that’s important. I think that the geriatric assessment can be helpful, as well.
There’s an IMWG [International Myeloma Working Group] calculator on the website that can give you a score, fit or not fit. We participated in a study where that was part of the assessment, and it sometimes surprised me. I thought I was pretty good at knowing what fitness my patients had ahead of time, but it was humbling to realize things that I had, maybe, not fully appreciated [their fitness] in my relationship with the patient. So, I find that the geriatric assessment, or scales like that, can sometimes be helpful.
What are your thoughts on the results of the poll? What would you have chosen?
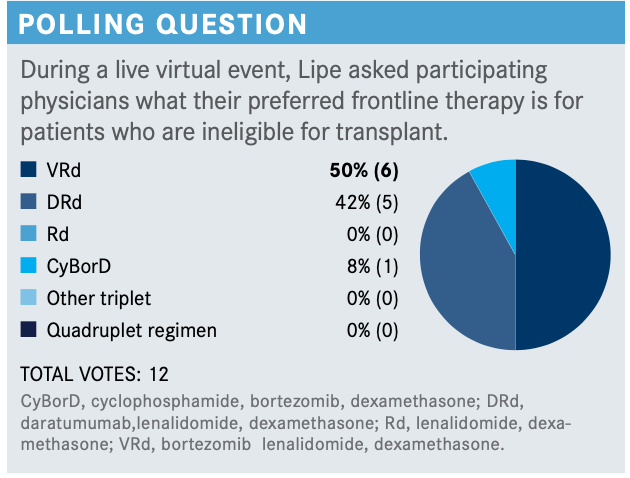
I’ve started using a lot of DRd [daratumumab (Darzalex), lenalidomide (Revlimid), dexamethasone]. I have used VRd [bortezomib (Velcade), lenalidomide, dexamethasone] Lite, or I’ve used VRd Lite plus daratumumab, but I worry about the bortezomib in the elderly patients because of the neuropathy and the falls. Several analyses looked at the use of bortezomib in the elderly population and showed increased rates of fall and worse outcomes related to fall. I worry about that in my truly frailer patients. I have used doublets in that setting; adding daratumumab doesn’t add a lot of toxicity but allows me to give them better therapy. So I do use a lot of DRd.
If you’re using VRd up front, do you give it continuously until progression, or until adverse events (AEs)?
Yes, that’s what I’ve done, until their neuropathy gets so bad that I stop it, or until they get any neuropathy. I have a pretty low threshold to drop the bortezomib in elderly patients.
What factors do you consider when choosing an induction regimen for a patient like this?
There are patient characteristics, there are disease characteristics, and there are therapy-related factors. I think the heterogeneity of this patient population and the unique situations requires an individualized therapeutic plan. And, frankly, this is one of the things I like most about my job—is getting to know these people and being able to tailor the therapies to what is right for them in a way that works for them best.
What trials and data support the frontline treatment options available in patients who are ineligible for transplant?
Some of the standard ones you think about are MAIA [NCT02252172], which is DRd versus Rd; the ALCYONE trial [NCT02195479]; VISTA [NCT00111319] looking at [bortezomib plus] melphalan and prednisone; the SWOG-S0777 trial [NCT00644228], which demonstrated improved overall survival [OS] for the triplet combination; VRd Lite [NCT01782963], and some of the data there; the FIRST trial [NCT00689936], which established Rd as the standard of care over MPT [melphalan, prednisone, thalidomide (Thalomid)]; and the CLARION trial [NCT01818752], which failed to demonstrate the benefit with carfilzomib [Kyprolis] [over bortezomib with melphalan and prednisone].
Comparing [some of] these trials—the overall response rates [were] pretty high overall, CR [complete response] rates were high across different trials, considering that we’re using largely triplets; [same with] progression-free survival [PFS].
Looking at the SWOG-S0777 study that looked at patients that were randomized to RVd versus Rd, patients on the lenalidomide/bortezomib/dexamethasone induction arm, followed by the Rd maintenance, did better in terms of PFS [41 vs 29 months; HR, 0.742; 96% CI, 0.594-0.928; P = .003] and OS [not reached vs 69 months; HR, 0.709; 96% CI, 0.543-0.926; P = .0114].1,2 This highlights the issues that we’re having in multiple myeloma. It took about 6 to 7 years to read out the OS advantage. So, it took a long time to demonstrate the benefit of the triplet versus the doublet, which I think just speaks to the efficacy of our newer regimens, and how it is becoming increasingly hard to demonstrate the OS advantage, and really, why we need better secondary end points.
At a median follow-up of 84 months, the triplet outperformed the doublet.
Speaking to the toxicity, as one would expect, there were higher rates of neuropathy on the triplet arm, [whereas] sensory issues and GI [gastrointestinal] issues were worse on the triplet arm.
What was different about the VRd Lite regimen and study?
This has been looked at in a couple of studies. There was a single-arm study that initially [explored the use of] VRd Lite. This was a retrospective analysis of chart reviews, looking at patients that were treated with VRd Lite.3 This is on a 28-day schedule, and it showed some reasonable response rates. The initial trial that was VRd Lite, published by Elizabeth K. O’Donnell, MD, in Boston, showed a PFS of 35.1 months.4
What about the DRd regimen?
The MAIA study—also another transplant-ineligible, newly diagnosed trial—looked at patients and randomized them to Rd versus DRd and showed the primary end point was improved for the daratumumab-containing arms. So improved PFS, not reached yet in the DRd arm versus 31.9 months for the Rd arm [HR, 0.56; 95% CI, 0.43-0.73; P <.0001].5 OS was not reached in either arm [HR, 0.78; 95% CI, 0.56-1.1], which again highlights how long these patients are living, even with the doublet therapy. So median follow-up at 28 months, and median OS is not reached. Which is to the point that sometimes a doublet is appropriate in these elderly patients. For some of my patients, if we had them still surviving 28 months, unrelated to their myeloma, that would be a positive end point for them.
Depth of response and PFS was improved in the daratumumab-containing arm. MRD negativity was improved in the DRd arm, and those patients who are MRD negative do better versus those who are MRD positive. MRD negativity appears to be the greater end point, because whatever arm they were on, they did better with MRD negativity.
Looking at the AEs in this trial, some of the hematologic toxicity, particularly the neutropenia, was worse for the daratumumab-containing arm. And then, some of the others—infusion-related reactions, some of the diarrhea, fatigue—[were all] increased for the daratumumab-containing arm. Respiratory infections were worse. What you’d expect with daratumumab.
How do you choose among these triplet options?
The PEGASUS analysis [looked at] patients who were treated in the Flatiron database, which is apparently a national treatment database for patients in community oncology practices, and they extracted patients who’d been treated with Rd versus VRd or Vd, and they tried to match these patients to patients in the MAIA trial, and once they found these patients, they found that patients who got the daratumumab-containing regimen, DRd, did better than either Rd alone [HR, 0.54; 95% CI, 0.42-0.71], or VRd [HR, 0.68; 95% CI, 0.48-0.98], and Vd [HR, 0.48; 95% CI, 0.33-0.69], as well.6
Looking at the analysis of the PFS [in the] patients out of the Flatiron database [with the MAIA data], that’s how they determined that the daratumumab arm on the MAIA trial did better.
What other triplet options have been considered for this setting?
The ENDURANCE trial [NCT01863550] was eagerly anticipated, and fell flat, I think, but basically showed that when patients were treated with VRd versus KRd [carfilzomib, lenalidomide, dexamethasone] up front, there was really no difference in PFS.7
I think a couple of important things to note in this trial are that this was for standard-risk patients, so high-risk patients were excluded. Patients were then given lenalidomide maintenance alone. So this negative trial, was it related to the intrinsic activity of these compounds, or was it more related to the trial design? I think that’s something that we’re all coming to our own conclusions about. Patients were pretty well randomized. And again, this excluded the truly high-risk patient population.
The PFS [34.6 vs 34.4 months; HR, 1.04; 95% CI, 0.83-1.31; P = .74] and OS [HR, 0.98; 95% CI, 0.71-1.36; P = .92] curves were...superimposed. So, PFS was similar, and this is amongst all the subgroups that they examined [as well].
Looking at the treatment-emergent AEs, for the VRd arms versus the KRd arms, they saw a difference in toxicity. They had higher levels of dropout on the VRd arm, compared with the KRd arm, but the toxicity was really related to the bortezomib, or the carfilzomib. So higher rates of neuropathy that accounted for a larger proportion of dropout were seen in the bortezomib-containing arm, and then higher rates of cardiac and pulmonary, renal dysfunction were seen in the carfilzomib arm.
This is demonstrating that AEs were unique to the treatment arms, and the larger amount of dropouts were on the VRd arm, and that was largely related to peripheral neuropathy.
REFERENCES
1. Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527. doi:10.1016/S0140-6736(16)31594-X
2. Durie BGM, Hoering A, Sexton R, et al. Longer term follow-up of the randomized phase 3 trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell trans-plant (ASCT). Blood Cancer J. 2020;10(5):5
3. doi:10.1038/s41408-020-0311-83. Rodriguez C, Lantz J, Akbar F, Lantz L, Dressler E. Assessing efficacy and toler-ability of a modified lenalidomide/bortezomib/dexamethasone (VRd-28) regimen using weekly bortezomib in multiple myeloma. Presented at: 17th International Myeloma Workshop; September 12-15, 2019; Boston, MA. Abstract SP-036.
4. O’Donnell EK, Laubach JP, Yee AJ, et al. A phase 2 study of modified lenalidomide, bortezomib and dexamethasone in transplant-ineligible multiple myeloma. Br J Haematol. 2018;182(2):222-230. doi:10.1111/bjh.15261
5. Facon T, Kumar S, Plesner T, et al; MAIA Trial Investigators. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104-2115. doi:10.1056/NEJMoa1817249
6. Durie BGM, Kumar SK, Usmani SZ, et al. Daratumumab‐lenalidomide‐dexameth-asone vs standard‐of‐care regimens: efficacy in transplant‐ineligible untreated myeloma. Am J Hematol. 2020;95(12):1486-1494. doi:10.1002/ajh.25963
7. Kumar SK, Jacobus SJ, Cohen AD, et al. Carfilzomib or bortezomib in combi-nation with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell trans-plantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21(10):1317-1330. doi:10.1016/S1470-2045(20)30452-6

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More