Tepotinib Demonstrates Durable Benefit With High-Level MET Amplification
Tepotinib was able to provide clinically meaningful activity in patients with high MET amplification non–small cell lung cancer in the VISION study, according to Xiuning Le, MD, PhD.

Tepotinib (Tepmetko) provided clinically meaningful activity with durable responses in patients with non–small cell lung cancer (NSCLC) and MET amplification, as detected by circulating tumor DNA (ctDNA), according to updated and exploratory findings from cohort B of the VISION trial (NCT02864992).1
“We demonstrated that tepotinib was able to provide clinically meaningful activity in patients with high MET amplification non–small cell lung cancer,” said Xiuning Le, MD, PhD, an assistant professor of thoracic/head and neck medical oncology at The University of Texas MD Anderson Cancer Center in Houston, when presenting the findings at the International Association for the Study of Lung Cancer 2022 North America Conference on Lung Cancer.
The MET inhibitor tepotinib was granted an accelerated FDA approval on February 3, 2021, for the treatment of adult patients with metastatic NSCLC harboring MET exon 14 skipping alterations, based on findings from the VISION study.2 However, the National Comprehensive Cancer Network (NCCN) also recommends that tepotinib can be used in patients with high-level MET amplification.3
VISION is an open-label, multicenter, phase 2 clinical trial consisting of 3 cohorts of patients with advanced NSCLC and MET exon 14 skipping alterations or MET amplifications.
In cohort B, patients had advanced NSCLC, a high-level MET amplification without a MET exon 14 skipping alteration according to the 73-gene Guardant360 liquid biopsy assay, and an ECOG performance status of 0 or 1. High-level MET amplification was defined as at least 2.5 gene copy numbers. “The ctDNA cutoff is different than [for] tissue, so 2.5 overall correlating with about 10 in the tissue,” Le noted.
Patients were treated in the first-, second-, or third-line setting and prior immunotherapy was allowed. All patients received 500 mg tepotinib once daily. The primary end point was objective response rate (ORR) by independent review committee per RECIST v1.1 criteria. Secondary end points included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
Exploratory biomarker analyses included a focus on co-amplification of up to 1 other chromosome 7 gene (EGFR, BRAF, or CDK6), co-occurring mutations and amplifications, ctDNA burden in maximum variant allele fraction (VAF) in each patient, and early molecular response in undetectable MET amplification after 6 to 8 weeks.
Among 24 patients in the cohort, the median age was 63.4 years, 88% were male, 88% were smokers, 88% had an ECOG performance status of 1, and 29% were treatment naïve.
In the overall cohort, the ORR was 41.7% (95% CI, 22.1%-63.4%), which included 1 complete response (CR) (4.2%), and the disease control rate (DCR) was 45.8% (95% CI, 25.6%-67.2%). The median DOR was 14.3 months (95% CI, 2.8-not evaluable [NE]), the median PFS was 4.2 months (95% CI, 1.4-15.6), and the median OS was 7.5 months (95% CI, 4.0-15.6).
Efficacy was particularly pronounced in the 7 patients treated in the first-line setting (TABLE1 ). The ORR and DCR was 71.4% (95% CI, 29.0%-96.3%), including 1 CR. The median DOR was 14.3 months (95% CI, 2.8-NE), the median PFS was 15.6 months (95% CI, 1.4-NE), and the median OS was 14.3 months (95% CI, 4.0-NE).
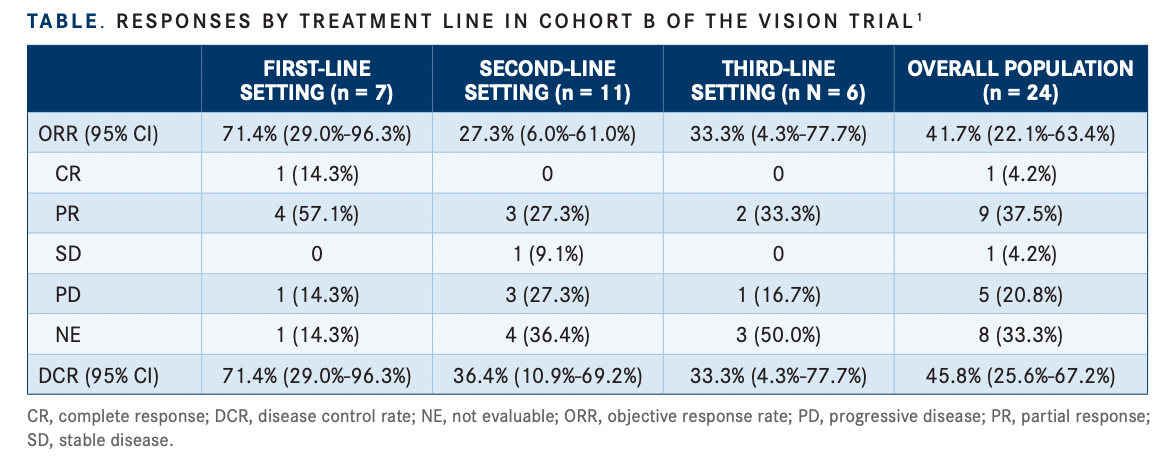
“The benefit is especially pronounced in the frontline setting for patients who are treatment naïve, we had observed a high response rate and a long duration of response,” Le said.
The median treatment duration was 3.6 months (range, 0.1-26.8) and 5 patients (21%) remained on treatment for at least a year, 2 of whom remained on treatment for at least 2 years (8%). One of these 2 patients achieved a CR.
The median PFS was highest in the firstline setting at 15.6 months, followed by 13.6 months in patients treated in the second-line setting and 1.7 months for patients treated in the third-line setting.
Exploratory Biomarker Analyses
Patients with focal MET amplification and absence of MYC/RB1 mutations were associated with improved outcomes. Patients who achieved clinical benefit, meaning a response or stable disease to treatment, were more likely to have focal MET amplification than those who did not achieve clinical benefit, meaning disease progression.
The most common co-occurring mutation was TP53 followed by CDK6 amplification, which appeared more in patients who did not achieve clinical benefit.
Median baseline ctDNA burden was 10.7% (interquartile range, 7.5%-26.0%). Patients with lower ctDNA burden than the median were associated with greater efficacy from tepotinib treatment. Patients with less tumor load were also more likely to achieve clinical benefit.
Investigators reported that early molecular response was achieved in 14 of 18 evaluable patients (77.8%), and these patients showed improved outcomes over patients without early molecular response.
Nine patients had available end-of-treatment biomarker profiles. Within this group, 2 patients (22.2%) had emergence of MET kinase domain mutations following treatment with tepotinib.
Patients with focal MET amplification showed greater ORR compared with those with non-focal amplification (57.1% vs 20.0%, respectively), according to researchers. Those with RB1 co-mutation did not show any benefit to treatment (0.0% vs 52.6%, respectively), nor did patients with MYC amplification (0.0% vs 55.6%). Patients with less ctDNA burden than the median (10.7%) had an ORR of 66.7% compared with 16.7% in those with greater-than-median ctDNA burden. Patients with early molecular response who responded to treatment showed an ORR of 71.4%.
Le noted that baseline focal MET amplification, RB1 wild-type status, MYC diploidy, and low ctDNA burden were potential predictors of clinical benefit from tepotinib treatment.
Confirmed Safety Profile
Treatment-related adverse events (TRAEs) of any grade were reported in 70.8% of patients, including most commonly edema (45.8%), peripheral edema (41.7%), generalized edema (16.7%), and constipation (16.7%), followed by transaminase increase, diarrhea, and hypoproteinemia, which was reported in 2 patients each (8.3%).
Grade 3 events were observed in 7 patients (29.2%), including edema and transaminase increase.
“In this cohort of patients, we observed very similar safety profile compared with what we already know about tepotinib, there [are] no new safety signals observed, there [are] no discontinuations due to TRAEs,” Le said.
REFERENCES
1. Le X, Paz-Ares LG, Meerbeeck JV, et al. Clinical Response to Tepotinib According to Circulating Tumor (ct)DNA Biomarkers in Patients with Advanced/Metastatic NSCLC with High-level MET Amplification (METamp) Detected by Liquid Biopsy (LBx). Presented at IASLC 2022 North America Conference on Lung Cancer; September 23-25, 2022; Chicago, IL.
2. FDA grants accelerated approval to tepotinib for metastatic nonsmall cell lung cancer. FDA. February 3, 2021. Accessed October 10, 2022. https://bit.ly/3STgtFo
3. NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. Version 5.2022. September 26, 2022. Accessed October 11, 2022. https://www.nccn.org/professionals/physician_gls/ pdf/nscl.pdf
