Sunvozertinib Shows Activity and Tolerability in EGFR Exon 20+ NSCLC
In patients with non–small cell lung cancer who harbor an EGFR exon 20 insertion mutation, sunvozertinib demonstrated promising efficacy.

Sunvozertinib (DZD9008) has demonstrated activity in patients with non–small cell lung cancer (NSCLC) harboring an EGFR exon 20 insertion mutation across mutation types and prior treatments, according to data pooled from 3 phase 1/2 clinical trials. Findings from the clinical trials were presented at the International Associationfor the Study of Lung Cancer 2022 North America Conference on Lung Cancer.1
“Sunvozertinib…has compelling clinical efficacy in patients with non–small cell lung cancer who received prior platinum [therapy] and have an EGFR exon 20 insertion,” Lyudmila A. Bazhenova, MD, said in a presentation of the updated findings at the conference.
Bazhenova is professor of medicine at Moores Cancer Center at UC San Diego Health in California.
Bazhenova noted that sunvozertinib is an EGFR exon 20 insertion inhibitor with selectivity for wild-type EGFR as well. The agent received a breakthrough therapy designation from the FDA and the Chinese National Medical Products Administration as a treatment for patients with NSCLC and EGFR exon 20 insertion mutation whose disease has progressed during or after platinum-based chemotherapy.2
Three clinical trials explored sunvozertinib, including WU-KONG1 (NCT03974022), an international phase 1/2 study with ongoing enrollment; WU-KONG2 (CTR20192097), a phase 1 study conducted in China; and WU-KONG6 (CTR20211009), a pivotal phase 2 study conducted in China. All patients in the 3 studies had locally advanced or metastatic NSCLC with an EGFR or HER2 mutation and adequate organ system function. Patients with brain metastases were allowed to participate in the study if the metastasis was stable. Treatment was administered in the studies at doses between 50 mg and 400 mg.
Pooled data included a total of 119 patients with an EGFR exon 20 insertion mutation in the efficacy analysis set and 238 patients in the safety analysis set. The median age of all patients was 58 (range, 29-85), 61.3% of patients were female, 89.1% of patients were Asian, and 63% of patients had an ECOG performance status of 1. Among all patients, 31.1% had brain metastasis at baseline. The median number of prior anticancer therapies was 2 (range, 1-10).
The recommended phase 2 dose was determined to be 300 mg of sunvozertinib. At this dose, the overall response rate (ORR) was 52.4% across studies. In the WU-KONG6 study, the confirmed ORR by blinded independent central review was 59.8%. Among patients with brain metastasis, the ORR was 44%.
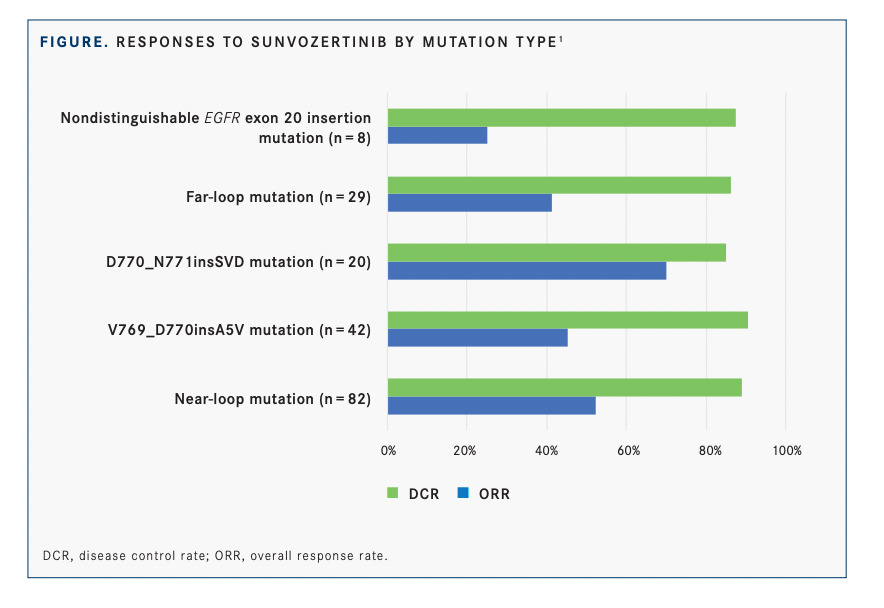
Responses were seen across various types of EGFR exon 20 insertion mutations, of which 30 kinds were confirmed in the efficacy analysis population (FIGURE1 ).
In patients with near-loop mutations, the most common type (n = 82), the ORR was 52.4% and disease control rate was 89%. In those with far-loop mutations (n = 29), the ORR was 41.4% and the disease control rate was 86.2%.
Additionally, responses were observed among 3 of 4 patients previously treated with amivantamab (Rybrevant).
In the safety analysis set, the most common treatment-emergent adverse events (TEAEs) reported were diarrhea (58%), rash (39.5%), blood creatine phosphokinase (CPK) level increase (29.4%), paronychia (27.7%), and anemia (26.5%).
Among the 169 patients who received the 300-mg dose of sunvozertinib, diarrhea was experienced by 59.2% of patients, rash by 39.1%, blood CPK level increase by 31.4%, anemia by 26.6%, and paronychia by 25.4%. “[Because] this drug is an EGFR tyrosine kinase inhibitor [TKI], we expect the [adverse events] to be similar to what we see in EGFR TKIs,” Bazhenova commented.
The majority of TEAEs were grade 1 or 2 in severity. The most common events of grade 3 or above in the overall population included blood CPK level increase in 9.2% of patients, diarrhea in 5.5%, anemia in 3.8%, and decreased appetite in 2.5%. At the 300-mg dose, blood CPK level increase of grade 3 or above was seen in 11.2% of patients, diarrhea in 6.5%, anemia in 3%, and decreased appetite in 1.8%.
REFERENCES
1. Bazhenova L, Jänne PA, Wang M, et al. Sunvozertinib in NSCLC patients with EGFR exon20 insertion mutations. Presented at: IASLC 2022 North America Conference on Lung Cancer; September 23-25, 2022; Chicago, IL.
2. FDA grants breakthrough therapy designation for Dizal Pharmaceutical’s DZD9008 in patients with locally advanced or metastatic nonsmall cell lung cancer harboring EGFR exon20 insertion. News release. Dizal Pharmaceutical. January 28, 2022. Accessed October 10, 2022. https://bit.ly/3ulggSf
