Cemiplimab Makes Case as First-Line, Nonchemotherapy Treatment in Metastatic NSCLC
Updated results of the EMPOWER-Lung1 trial of cemiplimab in patients advanced or metastatic non–small cell lung cancer showed improvements in overall survival, progression-free survival, and overall response rate.
After 3 years of follow-up, findings from the EMPOWER-Lung1 trial (NCT03088540) demonstrated further improvement in overall survival (OS), progression-free survival (PFS), and overall response rate (ORR) in patients with advanced or metastatic non–small cell lung cancer. Patients were given cemiplimab-rwlc (Libtayo) vs chemotherapy and continued to see improvements in OS despite a crossover rate of 75%, according to data presented by Mustafa Özgüroğlu, MD, during the International Association for the Study of Lung Cancer 2022 North America Conference on Lung Cancer.1
In the second part of the trial, patients were allowed to continue cemiplimab therapy with the addition of histologic-specific chemotherapy beyond progression; this resulted in durable responses and an ORR of 31.3%. In patients with PD-L1 greater than or equal to 50%, patients in the cemiplimab arm had a median OS of 26.1 months (95% CI, 22.1-31.8) vs 13.3 months in the chemotherapy arm (95% CI, 10.5-16.2) Risk of death was 43% (HR, 0.57; 95% CI, 0.46-0.71; P < .0001).
Similarly, PFS rates favored patients in the cemiplimab arm. Median PFS was 8.1 months in the treatment arm (95% CI, 6.2- 8.8) vs 5.3 months in the control arm (95% CI, 4.3-6.1). Risk of progression was 49% (HR, 0.51; 95% CI, 0.42-0.62; P < .0001).
ORR and duration of response also favored cemiplimab over chemotherapy with patients treated with cemiplimab with an ORR of 46.5% vs 21.0% in patients treated with chemotherapy (OR, 3.26; P < .0001).
Investigators reported that median duration of response (DOR) was reported as 23.6 months in the cemiplimab arm vs 5.9 months in the chemotherapy arm. Complete response (CR) was 8.1% (n = 284) in the cemiplimab arm compared with 2.1% (n = 281) in the treatment arm.
“The effect of PD-L1 levels was interesting,” said Özgüroğlu, professor of medical oncology, Istanbul University, Turkey. “Increases in ORR in patients given cemiplimab correlated with PD-L1 expression levels,” he continued.
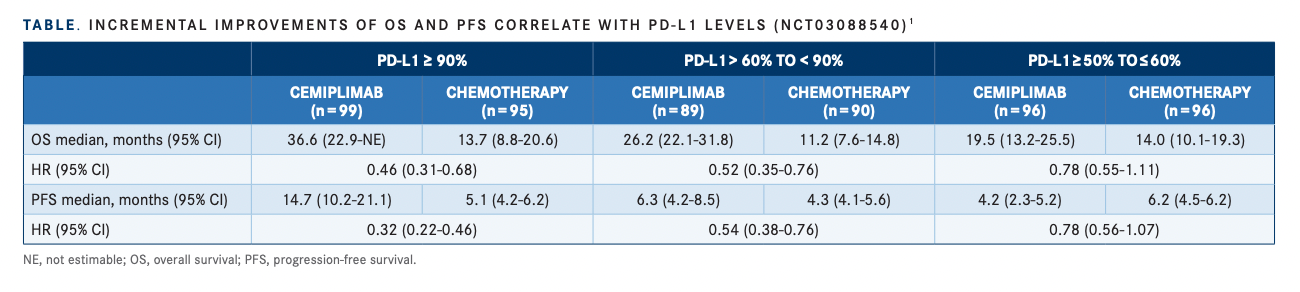
In patients with PD-L1 greater than or equal to 90%, ORR for patients who received cemiplimab (n = 99) was 60.6% vs 17.9% in patients who received chemotherapy (n = 95). For patients with PD-L1 levels between 60% and 90%, those who received cemiplimab (n = 89) had an ORR of 43.8% vs 22.2% for those who received chemotherapy (n = 90). For PD-L1 levels between 50% and 60%, ORR was 34.4% for those who received cemiplimab (n = 96) vs 22.9% for those who received chemotherapy (n = 96). Özgüroğlu reported similar findings for PFS rates and PD-L1 levels (TABLE1).
In the EMPOWER-Lung 1 trial, 721 patients in the intent-to-treat population were randomly assigned to receive 350 mg of cemiplimab every 3 weeks (arm A) vs 4 to 6 cycles of investigator’s choice for chemotherapy (arm B).
Eligible patients had treatment-naïve advanced NSCLC with PD-L1 levels greater than or equal to 50%, with an ECOG performance status of 0 or 1. The primary end points were OS and PFS, and secondary end points were ORR, DOR, and safety. At disease progression, patients in arm A could continue cemiplimab plus 4 cycles of chemotherapy. At disease progression, patients in arm B could cross over to receive cemiplimab monotherapy. A total of 565 patients had PD-L1 greater than or equal to 50% as determined by 22C3 assay.
In patients with PD-L1 greater than or equal to 50%, baseline characteristics were similar between the 2 arms. Median age was 63 years in the cemiplimab arm (n = 284) and 64.0 years in the chemotherapy arm (n = 281). The majority of patients for both arms were male (87.7% vs 82.2%, respectively).
“Safety profiles were consistent with previous studies involving cemiplimab,” noted Özgüroğlu. Any grade treatment-related adverse events (AEs) in the cemiplimab arm was 62.6% and discontinuation occurred in 7.3% in the treatment arm. Any grade treatment-emergent AEs were anemia (19.7%), decreased appetite (14.3%), and fatigue (13.8%). The most common grade 3-5 AEs were pneumonia (5.3%), anemia (4.2%), and dyspnea (2.8%).
After initial treatment with cemiplimab and disease progression, patients were given 4 cycles of chemotherapy (n = 64). After progression, Özgüroğlu said 3 patients had a complete response, 17 had a partial response, 35 patients had stable disease, and 9 patients progressed.
Improved PFS was reported after the addition of chemotherapy: at 6 months, the PFS rate was 50.7% in patients who received the initial monotherapy vs 66.2% after chemotherapy was added. Similarly, at 12 months, PFS was 24.1% vs 31.2%; at 18 months, 0.0% vs 15.7%; and at 24 months, 0.0% vs 8.4%, respectively.
“Overall, these results support the use of cemiplimab as a first-line, chemotherapyfree treatment for patients with advanced or metastatic NSCLC and PD-L1 greater than or equal to 50%,” Özgüroğlu concluded.
REFERENCE
1. Özgüroğlu M. Three-year outcomes per PD-L1 status and continued cemiplimab beyond progression + chemotherapy: EMPOWER-Lung 1. Presented at: 2022 North American Conference on Lung Cancer; September 23-25, 2022; Chicago, IL. Accessed October 11, 2022. Abstract OA01.05. https://bit.ly/3ECVmmy
