Targeting HRAS Mutations With Investigational Tipifarnib May Hold Promise in Some Tumor Types
The high variability in responses among different types of HRAS-altered cancers highlights the need to identify rational therapeutic combinations that enhance response to HRAS-directed therapies.
Alan L. Ho, MD, PhD

Tipifarnib, a highly potent and selective nonpeptide farnesyltransferase inhibitor (FTI), has shown highly durable responses in HRAS-mutant, heavily pretreated head and neck squamous cell carcinoma (HNSCC) that has failed to respond to other approved therapies. However, the high variability in responses among different types of HRAS-altered cancers highlights the need to identify rational therapeutic combinations that enhance response to HRAS-directed therapies.
HRAS is 1 of 3 human RAS proteins (along with KRAS and NRAS), which play important roles in proliferation, survival, and differentiation of cells and are commonly altered in several types of cancer.1 Alterations in the HRAS gene are present in approximately 0.97% of all cancers,1 with relatively high prevalence noted in urothelial carcinoma (4.42%),1 salivary duct carcinoma (13%-27%),2 and HNSCC (4%-8%).3 Presence of HRAS alterations has also been associated with poorer progression-free survival (PFS) in patients with HNSCC who received cetuximab (Erbitux), a targeted monoclonal antibody that inhibits EGFR, in the first-line recurrent or metastatic setting.4
Therapeutic Targeting of HRAS
Despite the high prevalence of RAS mutations in cancer, therapeutic targeting of RAS has been difficult in part because RAS proteins with activating mutations do not have increased enzymatic activity that can be inhibited, said Alan L. Ho, MD, PhD, in an interview with Targeted Therapies in Oncology. Ho indicated that novel approaches for targeting RAS are needed.
“Many of the successes of targeted therapy have been [with] drugs targeting enzymatic activity of certain oncogenes, so pharmacologically developing approaches to inhibit RAS activity, even though that seems like a logical step for treatment of human cancers, has been difficult,” said Ho, who is the Geoffrey Beene Junior Faculty Chair at Memorial Sloan Kettering Cancer Center in New York.
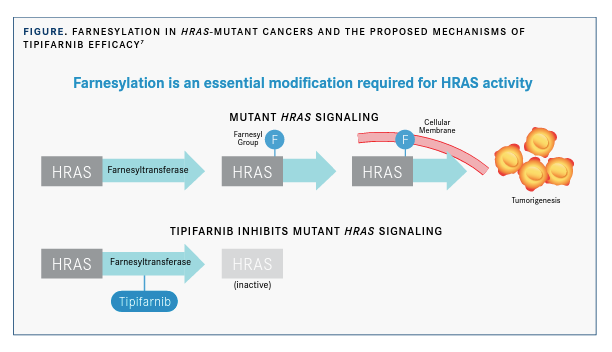
Effective RAS signaling requires attachment of the RAS proteins to the inner surface of the plasma membrane. Farnesyltransferase, a cytosolic metalloenzyme, initiates this process by transferring a 15-carbon hydrophobic moiety to the sulfhydryl group of cysteine in the RAS protein’s C-terminal CAAX motif. This process, farnesylation, plays an important role in the cellular membrane association and activity of RAS and other signaling proteins associated with cancer progression5; therefore, inhibition of farnesyl-transferase has been of clinical interest for the treatment of RAS-driven cancers.
Tipifarnib
Tipifarnib was the first selective FTI to be studied in clinical trials, beginning in 1997, but it showed only modest activity in KRAS- and NRAS-altered tumors. Results of subsequent studies revealed that KRAS and NRAS can undergo an alternative form of prenylation mediated by geranylgeranyl transferase that rescues these proteins from the membrane dis-placement caused by FTIs.6 However, HRAS is not a substrate for geranylgeranyl transferase, and FTIs were shown to suppress the membrane localization and downstream signaling of HRAS in human cancer cell lines expressing a mutant form of HRAS (FIGURE).6,7 These findings suggest a potential role for tipifarnib in HRAS-altered cancers, according to Ho.
“HRAS is unique in that it is solely dependent upon farnesyltransferase to localize to the membrane,” Ho said. “We have in vitro and some in vivo studies from our institution demonstrating that HRAS-driven cancers are particularly susceptible to FTIs.”
Head and Neck Squamous Cell Carcinoma
This distinctive characteristic of HRAS-driven tumors, along with the increased availability of tumor genotyping to identify tumors that may benefit from FTIs, has revived interest in tipifarnib as a possible treatment option for HRAS-altered cancers. The original protocol for the phase 2 KO-TIP-001 trial (NCT02383927) included a cohort of patients with HRAS-mutant thyroid cancer (cohort 1) and one with HRAS-mutant nonthyroid solid tumors (cohort 2).8
Preliminary results presented at the European Society for Medical Oncology 2018 Congress showed that tipifarnib was well tolerated and induced a partial response (PR) in 5 of 6 patients (83%) with locally advanced or met-astatic HNSCC, none of whom had responded to prior lines of therapy, including platinum chemotherapy, immunotherapy, and cetuximab with or without chemotherapy.8
The study also demonstrated a relationship between high mutant-HRAS variant allele frequency (VAF) and response to tipifarnib, which prompted amendment of the protocol to discontinue enrollment of the thyroid cancer cohort (because of low response rates), limit cohort 2 to patients with HNSCC with a mutant-HRAS VAF of 20% or greater, and add a third cohort of patients with squamous cell carcinomas (other than HNSCC) harboring HRAS mutations.9
Ho, the first author on the study, explained the rationale for narrowing the focus to patients with HNSCC. “Along the way, we discovered that there was a particularly good signal in [patients with] HRAS-mutant head and neck cancer,” he said. “Because sometimes you only get limited shots at demonstrating activity of these approaches, the decision was made to focus that second cohort of [patients with] solid tumors entirely on [patients with] head and neck cancer to fully delineate the true signal of tipifarnib in HRAS-mutant solid tumors.”
Ho added that the starting dose was also reduced from 900 mg to 600 mg, taken twice daily on a 1-week-on, 1-week-off schedule, to improve tolerability and help patients maintain an effective dose for as long as possible.
Interim results for the HNSCC cohort, which were presented at the 2019 American Association for Cancer Research/National Cancer Institute/European Organisation for Research and Treatment of Cancer International Conference on Molecular Targets and Cancer Therapeutics, included an objective response rate (ORR) of 56% in 18 evaluable patients, most of whom were pretreated, with a median of 2 prior therapies (range, 0-6).8 Responses were durable, and the PFS was numerically higher with tipifarnib than with the patient’s last prior therapy (6.1 vs 2.8 months). The treatment was generally considered tolerable, with the most common grade 3 or higher treatment-emergent adverse events of anemia (35%), neutropenia (20%), leukopenia (10%), thrombocytopenia (10%), and nausea (10%) adequately managed with supportive care.
“All of the treatment-emergent adverse events that we’ve seen are consistent with the known [adverse] effect profile that’s been previously delineated in past phase 2 and 3 trials,” said Ho.
An analysis of the HNSCC cohort presented at the 2020 American Society of Clinical Oncology Virtual Scientific Program showed an ORR of 50% (95% CI, 26.0%-74.0%), with a median duration of response (DOR) of 14.7 months, a PFS of 5.9 months, and an overall survival (OS) of 15.4 months. By contrast, 3 FDA-approved therapies for second-line treatment of HNSCC have ORRs of 13% to 16%, a median PFS of 2 to 3 months, and a median OS of 5 to 8 months.10
“We believe a median overall survival of 15 months is unprecedented in this patient population and represents a substantial improvement compared [with] historical bench-marks with current standard of care,” said Troy Wilson, PhD, JD, president and CEO of Kura Oncology, a biopharmaceutical company in San Diego, California, in a press release.11 “Taken together, these data support our enhanced focus on patients with HRAS-mutant HNSCC, a population in desperate need of effective new treatments, and reinforce our confidence in the ongoing AIM-HN registration-directed trial.”
This international phase 2 trial (AIM-HN/SEQ-HN; NCT03719690) was initiated in response to the KO-TIP-001 results in patients with HNSCC. The AIM-HN cohort is evaluating the efficacy of tipifarnib in HRAS-mutant HNSCC, and the SEQ-HN cohort is an observational substudy evaluating the effect of HRAS mutations on response to first-line HNSCC therapies. The primary outcome measure is ORR, and secondary outcome measures include DOR, time to response, OS, and PFS.
Salivary Gland and Urothelial Cancers
Although early results suggest promising efficacy of tipifarnib in HNSCC with a high mutant-HRAS VAF, reports suggest more modest efficacy in other tumor types harboring HRAS alterations. A recent analysis of patients with salivary gland cancer who were in the original cohort 2 of the KO-TIP-001 trial (n = 7), or who received tipifarnib under individual patient FDA investigational new drug application or single patient expanded access requests (n = 6), showed that of 12 evaluable patients, 1 had a PR and 7 had stable disease (SD) as best response.11 The median duration of SD was 9 months, with SD continuing for at least 6 months in 6 of the 7 patients.12 The median PFS and OS were 7 months (95% CI, 5.9-10.1) and 18 months (95% CI, 9.6-22.4), respectively.11
In the phase 2 IST-01 trial (NCT02535650), which included patients with refractory metastatic urothelial carcinoma harboring missense, nonsynonymous HRAS mutations (n = 14) or the rs2075606 (T>C) single-nucleotide variant (SNV) in the tumor suppressor gene STK11 (n = 7), tipifarnib produced ORRs of 24% in the intention-to-treat analysis and 42% in the subgroup of patients with HRAS-mutant tumors. Four patients (19%) achieved PFS at 6 months, including 3 patients with missense HRAS mutations and 1 patient enrolled as an STK11 SNV carrier who also had a nonsense mutation in HRAS. The study authors recommended further studies evaluating the addition of agents, such as immune checkpoint inhibitors, to tipifarnib to improve outcomes in urothelial carcinoma.13
“Just based on the response rates, there’s a range of activity of tipifarnib demonstrated against HRAS-mutant malignancies based on lineage, with [patients with head and neck cancer] showing the best activity,” said Ho. “The reasons for these disparities are not known and are a subject of investigation right now on the preclinical side.”
Results of one preclinical study showed that although tipifarnib led to tumor regression and improved survival in mice bearing HRAS-driven poorly differentiated and anaplastic thyroid tumors, early and late resistance to tipifarnib was observed. Whole-exome sequencing of several resistant tumors identified a nonsense mutation in NF1 and an activating mutation in GNAS at high allele frequencies. The investigators noted that these gene alterations drive acquired resistance to tipifarnib by reactivating RAS/MAPK downstream signaling and concluded that this finding supports the drug’s on-target effects.14
“The natural question becomes: [Are] the differences in responses or clinical activities related to tumor-specific differences in the biochemical responses or feedback or increased likelihood of resistance…or [is it] simply [because] HRAS plays different roles in the different tumor types?” said Ho. “These are all possibilities that are being examined preclinically. What we know from the clinical data is that there’s a range of activity that is not entirely explained based on HRAS-mutant status.”
Future Directions
According to Ho, further study of tipifarnib in larger groups of patients and development of rational therapeutic combinations are important steps to identify the role of tipifarnib in [treating] HRAS-driven cancers. In one study, liquid biopsies identified acquired activating HRAS, KRAS, or NRAS mutations after cetuximab exposure in more than one-third of patients with HNSCC, whereas HRAS mutations were observed in only 4.3% of patients with cetuximab-naïve HNSCC. The study results also showed acquired RAS mutations in 46% of patients with disease progression during cetuximab treatment, whereas no RAS mutations were identified in patients without progression.15
Ho said that although these observations need further study, they suggest that combining cetuximab with an agent that targets RAS activation may be an approach to prevent or delay emergence of acquired resistance or to overcome established resistance to cetuximab. He also said that sequencing tumors of patients who have progressed on cetuximab may identify patients with HRAS mutations who could benefit from HRAS-directed therapy.
As for combination therapy, Ho said that although cetuximab is the most obvious addition to study with tipifarnib, the recent incorporation of immune checkpoint blockade into standard-of-care options for HNSCC raises the question of whether such agents could be combined safely and effectively with tipifarnib.
Ho concluded that increased clinical trial participation and identification of patients eligible for HRAS-directed therapy through next- generation sequencing will be essential to identify patients most likely to benefit from tipifarnib and clarify the differences among tumor lineages and HRAS gene alterations that influence responsiveness to tipifarnib.
“I encourage people to get next-generation sequencing for head and neck tumors and, if [they have] HRAS mutations, get them referred to clinical trials so that we can fully understand the activity and safety and, hopefully, get [tipifarnib] into standard treatment,” said Ho.
References:
1. HRAS. My Cancer Genome. Accessed September 7, 2020. https://bit. ly/2F0zR2N
2. Nakaguro M, Tada Y, Faquin WC, Sadow PM, Wirth LJ, Nagao T. Salivary duct carcinoma: updates in histology, cytology, molecular biology, and treatment. Cancer Cytopathol. Published online May 18, 2020. doi:10.1002/cncy.22288
3. Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576-582. doi:10.1038/nature14129
4. Leblanc O, Vacher S, Lecerf C, et al. Biomarkers of cetuximab resistance in patients with head and neck squamous cell carcinoma. Cancer Biol Med. 2020;17(1):208-217. doi:10.20892/j.issn.2095-3941.2019.0153
5. Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17(11):3631-3652. doi:10.1200/JCO.1999.17.11.3631
6. Whyte DB, Kirschmeier P, Hockenberry TN, et al. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem. 1997;272(22):14459-14464. doi:10.1074/jbc.272.22.14459
7. Ho AL, Chau N, Bauman J, et al. Preliminary results from a phase 2 trial of tipifarnib in squamous cell carcinomas (SCCs) with HRAS mutations. Ann Oncol. 2018;29(suppl 8):5432. doi:10.1093/annonc/mdy287.002
8. A farnesyltransferase inhibitor study. Kura Oncology. Accessed September 28, 2020. https://bit.ly/2Gf3MV1
9. Ho A, Brana I, Haddad R, et al. Preliminary results from a phase 2 trial of tipifarnib in head and neck squamous cell carcinomas (HNSCCs) with HRAS mutations. Abstract presented at: American Association for Cancer Research/National Cancer Institute/European Organisation for Research and Treatment of Cancer International Conference on Molecular Targets and Cancer Therapeutics; October 26-30, 2019; Boston, MA.
10. Ho AL, Hanna GJ, Scholz CR, et al. Preliminary activity of tipifarnib in tumors of the head and neck, salivary gland and urothelial tract with HRAS mutations. J Clin Oncol. 2020;38(suppl 15):6504. doi:10.1200/ JCO.2020.38.15_suppl.6504
11. Kura Oncology reports overall survival data from phase 2 trial of tipifarnib in HRAS mutant head and neck squamous cell carcinoma. News release. Kura Oncology Inc. May 29, 2020. Accessed September 8, 2020. https://bit.ly/2FkfwoB
12. Hanna GJ, Guenette JP, Chau NG, et al. Tipifarnib in recurrent, metastatic HRAS-mutant salivary gland cancer. Cancer. 2020;126(17):39723981. doi:10.1002/cncr.33036
13. Lee HW, Sa JK, Gualberto A, et al. A phase II trial of tipifarnib for patients with previously treated, metastatic urothelial carcinoma harboring HRAS mutations. Clin Cancer Res. Published online July 7, 2020. doi:10.1158/1078-0432.CCR-20-1246
14. Untch BR, Dos Anjos V, Garcia-Rendueles MER, et al. Tipifarnib inhibits HRAS-driven dedifferentiated thyroid cancers. Cancer Res. 2018;78(16):4642-4657. doi:10.1158/0008-5472.CAN-17-1925
15. Braig F, Voigtlaender M, Schieferdecker A, et al. Liquid biopsy monitoring uncovers acquired RAS-mediated resistance to cetuximab in a substantial proportion of patients with head and neck squamous cell carcinoma. Oncotarget. 2016;7(28):42988-42995. doi:10.18632/oncotarget.8943
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More