Optimizing Benefit in Targeted Therapies Requires Multipronged Educational Approach
Much is still unknown about the cancer process and how targeted therapies work on specific tumor vulnerabilities. Providers must closely follow evolving information, especially regarding adverse events.
Robert Coleman, MD

Oncology is rapidly transitioning into a field where tumor types are increasingly sub-segmented by molecular characteristics, and treatments are becoming structurally informed and designed based not only on tumor characteristics but also patient characteristics. In the conduct of tumor sequencing, multiple alterations are often discovered. However, alignment between molecular alteration and treatment efficacy is still imperfect. Consequently, it is common to use several drugs aimed at various alterations, rather than just 1. Although this approach may be necessary, it increases the complexity of treatment and the possibility of adverse events.
Sarah Alwardt, PhD

Much is still unknown about the cancer process and how targeted therapies work on specific tumor vulnerabilities. Providers must closely follow evolving information, especially regarding adverse events. This knowledge will enable them to be proactive to quickly modify treatment and avoid compromising efficacy while keeping the patient on therapy.
Adverse Effects Differ From Chemotherapy
Chemotherapy as a treatment modality has been used since the 1940s, so its adverse effects are generally well known. Adverse effects caused by targeted therapies can be different from chemotherapy. Some adverse effects are still under appreciated because, in the context of a clinical trial report, they were not fully described due to low frequency and/or prevalence; however, that does not mean that they are harmless. When a targeted agent combines with other drugs the patient is taking, an unexpected adverse effect may appear and be more severe than those caused by chemotherapy.
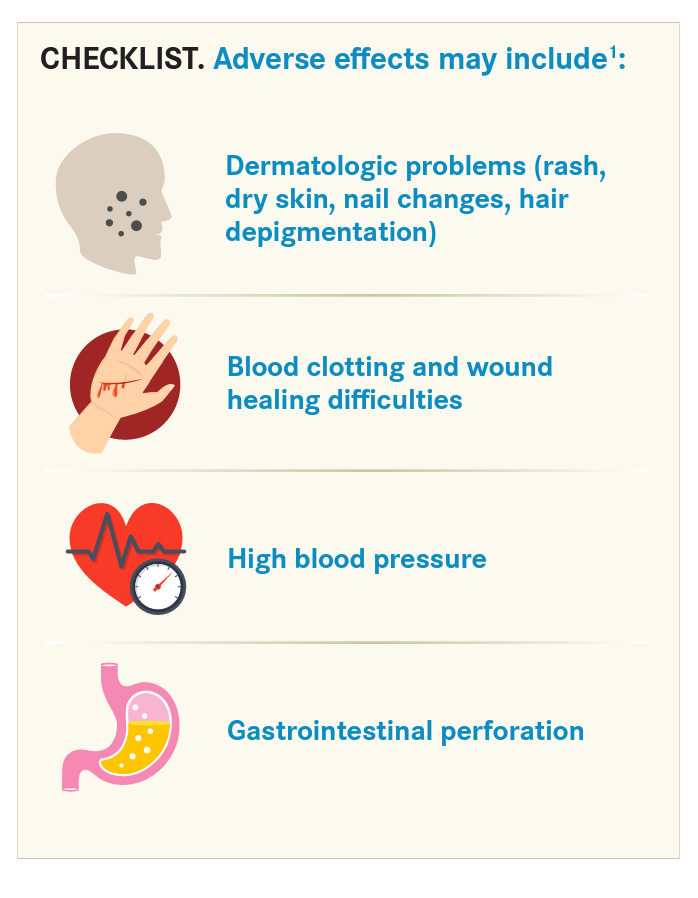
Many immunotherapy agents can trigger serious problems, such as autoimmune-based adverse events. Providers must respond quickly to manage these conditions. Some of these adverse effects, such as thrombocytopenia, leukopenia, or anemia, may not require management or can be handled in the office. However, a patient with severe diarrhea or pneumonitis who is taking a checkpoint inhibitor can quickly be in a life-threatening situation that requires hospitalization.
Antibody-drug conjugates (ADCs) are an example of how adverse effects of a parent compound might be mitigated while new issues arise. ADCs were developed to provide a “targeted” approach to delivering chemotherapy drugs that might otherwise be too toxic to be systemically delivered in its native form. The conjugates leverage tumor cell expression of antigens and/or receptors to focus delivery of a cytotoxic warhead. For the most part, these conjugates are stable in solution and only release their active agent upon binding and internalization at the cancer cell level. However, unique toxicities may occur due to normal cell expression of the same targets or irritation from the conjugate itself.
Today, there are many different types of targeted drugs, and the knowledge base is still being developed concerning adverse events they may cause. Adverse effects largely depend on the type of drug administered and what it targets. The most common adverse effects associated with certain targeted therapies are diarrhea and liver problems, such as hepatitis and elevated liver enzymes (CHECKLIST).1
Clinical Trials Leave Many Questions Unanswered
Even though new targeted therapies may have received FDA approval, little is known about their performance and safety in the broader patient population. Approval in a clinical trial does not mean the drug is right for everyone. Trial populations are relatively small compared with the prevalent population of patients, and documented adverse events may be similarly challenged–particularly since the manner in which adverse events are reported in the medical literature highlights the most common events (usually ≥10%) and the highest degrees of severity. This underestimates the potential burden of less common and less severe adverse events that could have a major impact on treatment tolerance. Extrapolating that data to the real world leaves many unanswered questions. With so many unknowns, bringing these drugs to a broader population is challenging and requires surveillance–a process called pharmacovigilance.
A major difference in a clinical trial’s study population versus the general at-risk population is how the patients on a trial were selected for participation. To reduce the number of confounding variables that could affect the primary efficacy signal, eligibility criteria are outlined to homogenize the study participants. While eliminating patients with preexisting conditions provides a clearer signal, it also effectively reduces extrapolation value, bringing unknown risks to patients not matching those used for the study. Most drug approvals carry a postmarketing commitment to gather data on real-world evidence, and updated safety packages are provided as new data become available. In some cases, this has led to more restrictive general use because the adverse events were more common than anticipated.
Although drug manufacturers do a good job of compiling data about adverse events, much is still unknown. Simply put, health care providers can find themselves in uncharted waters, trying to navigate the safest way for patients to receive potentially lifesaving therapies with limited information. Providers must understand that targeted therapies can potentially affect normal processes in the body and create adverse effects that may be unknown or under appreciated at the time of study closure. Consequently, providers need to be on alert for all symptoms, even if these symptoms are vague or unattributed, as they may ultimately signal the start of an escalating problem.
Comorbidities Present Challenges
Comorbidity is very common among patients with cancer. Most commonly, it’s the rule rather than the exception. Among the top 4 incident cancers–lung, colorectal, breast, and prostate comorbidity rates are 52.9%, 40.7%, 32.2%, and 30.5%, respectively.2 These secondary illnesses must be managed to ensure patients are healthy enough to withstand treatment. However, it is well documented that some of these medications can complicate the effective delivery of cancer medicines, particularly drugs where natural metabolism is shared.
Continuing education at the provider level is crucial to the effective and safe management of patients with comorbid conditions. Detailed history of concomitant disease and current medication is essential to developing a comprehensive treatment plan. Increasing complexity of both the oncologic and comorbid condition treatment heightens the need for providers to continually update their understanding of new agents and adverse events, utilizing educational opportunities that focus on these new therapies.
Mitigating strategies can be developed based on data that emerge as therapies become more widely used in patients with comorbidities. This approach has been successful with immunotherapy, especially CAR (chimeric antigen receptor) T-cell therapy. As the severity of the adverse effects began to emerge, toxicity guidelines were developed to help providers manage severe autoimmune adverse events, with special attention paid to patients with comorbidities. Today, providers can receive accreditation for delivering CAR T-cell therapy and other complex therapies. As more advanced treatments appear, accreditation may become a reimbursement requirement.
Enabling Successful Treatment Providers can help ensure optimal outcomes for patients undergoing targeted therapies by employing best practices in several key areas, such as:
Identifying possible adverse events
Patients and providers should be thoroughly educated about known adverse effects. Pharmaceutical companies have done a good job producing materials that provide pretreatment education concerning adverse effects and when to seek help if experiencing difficulties. With immunotherapies, it is critical for patients and providers to understand when symptoms must be immediately addressed and when they can wait. Certain symptoms can worsen in a matter of hours, creating a life-threatening situation.
Monitoring patients
Closely monitoring patients enables providers to be proactive about adverse events. Many clinical trials today are actually measuring the time to an adverse event and developing a profile for the drug under study. This is a giant step forward in managing symptoms, as it enables providers to create mitigation strategies and understand the natural cadence of adverse events.
There are several approved targeted drugs that require close monitoring because they can cause severe effects outside of the office between cycles, such as hypertension. When this was recognized during trials, home blood pressure cuffs and logs were provided to patients to track their readings. Utilizing this information, a combination therapy was identified that created dangerous adverse effects within the first 2 weeks, enabling providers to proactively manage these symptoms. When the drugs were finally approved, these best practices were brought to the office level to mitigate adverse effects.
Certain PARP inhibitors also require closer monitoring, particularly for platelets and bone marrow suppression. Consequently, weekly counts after the first treatment cycle are required with some of these agents to identify potential problems.
Many practices in The US Oncology Network are using triage and symptom tracking via patient portals to assess degrees of urgency. This real-time communication helps ensure patients get immediate care if needed, minimizing adverse events.
Staying Current Is Challenging But Critical
The rapid pace of emerging targeted therapies is creating a dynamic world that is increasingly complex for providers. Keeping up with the huge volume of information required to offer these therapies is difficult to manage in addition to patient care responsibilities. Undoubtedly, this will be an ongoing challenge.
The key to success may lie in using all forms of instructive technology and presentations focused on targeted therapies, including social media, webinars, publications, seminars, and other offerings. These educational opportunities enable providers to share real-world evidence and experiences, helping to build a critical knowledge base. As more new agents emerge, every available avenue should be utilized to keep the nuances of these complex therapies top-of-mind for providers so they can deliver safe, expert care.
References:
1. Targeted cancer therapies. National Cancer Institute. Accessed September 14, 2020. https://bit.ly/2ZQohyl
2. Pirschel C. The impact of comorbidities on patient care. ONS Voice. January 30, 2017. Accessed September 14, 2020. https://bit.ly/35Ahe0v
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More