Expert Reviews Novel Strategies in the Pipeline for the Treatment of ER-Positive Breast Cancer
Recent acceleration in the introduction of new regimens for the treatment of estrogen receptor–positive breast cancer has led to significant survival enhancement, but questions remain regarding how patients should be stratified following disease progression with these therapies.
William J. Gradishar, MD

Recent acceleration in the introduction of new regimens for the treatment of estrogen receptor (ER)–positive breast cancer has led to significant survival enhancement, but questions remain regarding how patients should be stratified following disease progression with these therapies.1
“In the last 7 or 8 years, we’ve seen the CDK4/6 inhibitors added to endocrine therapy and most recently the addition of alpelisib [Piqray], a PI3K inhibitor for patients who have that mutation present in their tumor,” William J. Gradishar, MD, said during a presentation at the 22nd Annual Lynn Sage Breast Cancer Symposium. “Nonetheless, we still have patients who develop progressive disease, and new strategies are needed.”
Looking to the latest version of the National Comprehensive Cancer Network guidelines for the treatment of breast cancer,2 Gradishar, who served as the meeting chair, noted that the newest regimens, including mostly combination therapy approaches with CDK4/6 inhibitors, are those with the highest level of recommendation for use in patients with ER-positive disease.
“Whereas 10 years ago we might have seen a series of monotherapy [regimens], now in the first- and second-line settings, we typically have treatments that combine endocrine therapy with either a CDK4/6 inhibitor, an mTOR inhibitor after progression on a CDK4/6 inhibitor, or, for those patients who have a PI3K mutation, the addition of alpelisib,” he said.
Gradishar, who is also a professor of medicine (hematology and oncology) and chief of hematology and oncology in the Department of Medicine at Northwestern University Feinberg School of Medicine in Chicago, Gradishar, Illinois, said a better understanding of disease biology has led to the addition of these agents to the backbone of endocrine therapy. More than 30 years ago, a simplistic view of breast cancer existed, in which estrogen interacted with tumor cells by combining with the estrogen receptor. With the introduction of hormonal therapy like tamoxifen, which blocked the effects of estrogen, tumor growth could be shut off. Other drugs, including aromatase inhibitors and estrogen receptor downgraders, were introduced and indirectly affected tumor growth.
“The big issue is: Can we continue CDK4/6 inhibitors, do we have other strategies to overcome resistance to these drugs, and how do we approach them?” Gradishar said.
“When we think about how we approach [breast cancer] today, it’s based on a better understanding of what goes on in the cell, particularly when resistance begins to develop,” Gradishar said. “We now appreciate that you don’t have to have only ligand binding to influence the growth of the cell. You can have ligand independent growth, and that is stimulated by signaling pathways such as the PI3K/AKT/mTOR and CDK4/6.”
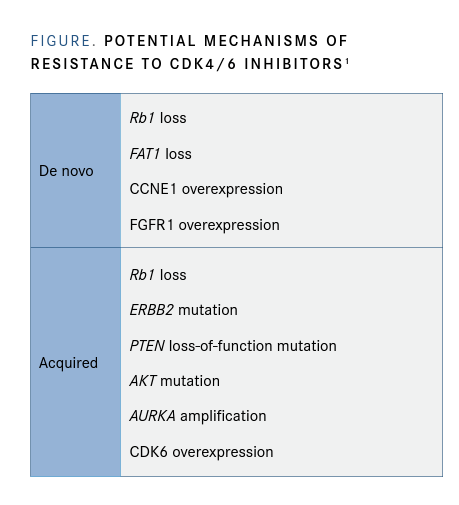
Strategies Under Investigation CDK4/6 inhibitors interfere with progression through the cell cycle. A number of reasons may explain why patients progress on CDK4/6 inhibitors and why the resistance develops. In the de novo setting where patients simply do not respond, it may be due to a loss of the tumor suppressor gene, Rb; amplification of FAT1 via the Hippo pathway; or overexpression of cyclin proteins. Acquired resistance following CDK4/6 inhibition has been linked with loss of Rb, mutations in ERBB2, PTEN loss-of-function mutations, AKT amplifications, Aurora A kinase amplification, and CDK6 overexpression (FIGURE).1
“There is a biology emerging that suggests there is a master regulator of the cell cycle, referred to as CDK7, and this may trump the effect of CDK4/6,” Gradishar said. “In this setting where patients progress, there is evidence that inhibitors of CDK7 may have an effect on overcoming resistance that develops.”
In preclinical models, the combination of fulvestrant and the CDK7 inhibitor CT7001 led to a decrease in tumor volume that was more pronounced than with either agent alone, suggesting synergy with the regimen. A phase 1b/2 trial is ongoing to assess the efficacy of fulvestrant with or without CT7001 in patients with ER-positive, HER2-negative breast cancer (NCT03363893).
Although ER-positive disease has been traditionally thought of as a cold tumor, Gradishar said preclinical data suggest adding immune checkpoint inhibitor therapy to CDK4/6 inhibition and endocrine therapy combinations may result in synergistic efficacy.3 The phase 2 PACE trial (NCT03147287) is a 3-arm trial for determining efficacy of fulvestrant, palbociclib (Ibrance), and avelumab (Bavencio). The triplet will be compared against arms of fulvestrant monotherapy and fulvestrant plus palbociclib.
Investigators are evaluating another strategy, adding mTOR inhibitors to endocrine therapy and CDK4/6 inhibition, in the phase 1/2 TRINITI-1 trial (NCT02732119) in postmenopausal patients who have progressed on endocrine therapy. In preclinical models, evidence shows that adding everolimus (Afinitor) to ribociclib (Kisqali) and exemestane in the setting of resistance may help to overcome these mechanisms.
Previously reported data from the trial showed that 33.4% of patients were progression free at 1 year, with a median progression-free survival (PFS) of 5.7 months with everolimus, ribociclib, and exemestane. The clinical benefit rate at 24 weeks was 41%.4
“It was also demonstrated that in patients who had either an estrogen receptor or a PI3K mutation, the overall [disease] behavior is much worse,” Gradishar said. “We can tease out from these populations patients who might do worse than others.”
Translational research related to pivotal trials MONALEESA-2 (NCT01958021) of ribociclib plus letrozole in postmenopausal women and PALOMA-3 (NCT01942135) of palbociclib plus fulvestrant for patients with disease progression following endocrine therapy indicates that FGFR1 amplification may be a marker of poor prognosis to CDK4/6 inhibitor treatment regimens.5,6
A number of trials are ongoing for drugs with activity against FGFR1. One such study is the phase 2 trial of fulvestrant plus dovitinib, a small molecule inhibitor of FGFR1, 2, and 3 as well as other receptor kinases (NCT01528345). Results indicate that with FGFR1 amplification, an efficacy advantage exists, evidenced by superior median PFS observed with dovitinib versus placebo (10.9 vs 5.5 months; HR, 0.64; 95% CI, 0.22-1.86).7
In the setting of Rb loss and CDK4/6 resistance, evidence shows that AURKA may be amplified. As a result, the phase 2 TBCRC 041 trial (NCT02860000) is exploring alisertib, an inhibitor of AURKA, alone or with fulvestrant to determine whether this strategy is feasible for overcoming resistance.
Selective Estrogen Receptor Degraders
In the setting of ER mutations such as ESR1, the potential for selective ER degraders (SERDs) came into focus. The only such agent with FDA approval is fulvestrant, which was approved in 2002.8
Development continues, and several clinical trials are currently exploring next-generation agents. Investigators are evaluating the oral SERD elacestrant in trials with previously reported data showing evidence of single-agent activity in heavily pretreated patients, including those with prior fulvestrant and CDK4/6 inhibitors as well as those with ESR1 mutations.9
Another SERD with activity in patients with and without ESR1 mutations is AZD9833, which the ongoing phase 1 SERENA-1 trial (NCT03616587) is exploring. Results presented this year indicate activity at all dose levels and in patients with prior CDK4/6 inhibitors or fulvestrant.10
Future Directions
Despite the efficacy of CDK4/6 inhibitors, patients do eventually have progression following such therapy, and sequencing strategies are needed to determine the best next steps in the evolution of the disease.
“As we go forward, we need to recognize… where we are with standard of care,” Gradishar said. “The data regarding the use of CDK4/6 inhibitors after progression is evolving with trials that are ongoing. Other strategies that include targeted therapies,…possibly immune therapy, and master regulators like the CDK7 inhibitors may offer new avenues for patients with progressive disease for patients with ER-positive metastatic breast cancer.”
References:
1. Gradishar WJ. Novel approaches for endocrine-resistant breast cancer. Presented at: 22nd Annual Lynn Sage Breast Cancer Symposium; September 10-12, 2020; virtual.
2. NCCN. Clinical Practice Guidelines in Oncology. Breast cancer, version 6.2020. Accessed September 21, 2020. https://bit.ly/3cgNQh4
3. Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471-475. doi:10.1038/nature23465
4. Bardia A, Hurvitz SA, DeMichele A, et al. Triplet therapy (continuous ribociclib, everolimus, exemestane) in HR+/HER2− advanced breast cancer postprogression on a CDK4/6 inhibitor (TRINITI-1): efficacy, safety, and biomarker results. J Clin Oncol. 2019;37(suppl 15):1016. doi:10.1200/JCO.2019.37.15_suppl.1016
5. O’Leary B, Cutts R, Huang X, et al. Genomic markers of early progression on fulvestrant with or without palbociclib for ER+ advanced breast cancer in the PALOMA-3 trial. J Clin Oncol. 2019;37(suppl 15):1010. doi:10.1200/JCO.2019.37.15_suppl.1010
6. Hortobagyi GN, Stemmer S, Campone M, et al. First-line ribociclib + letrozole in hormone receptor-positive, HER2-negative advanced breast cancer: efficacy by baseline circulating tumor DNA alterations in MONALEESA-2. Cancer Res. 2018;78(4)(suppl):PD4-06. doi:10.1158/15387445.SABCS17-PD4-06
7. Musolino A, Campone M, Neven P, et al. Phase II, randomized, placebo-controlled study of dovitinib in combination with fulvestrant in postmenopausal patients with HR+, HER2- breast cancer that had progressed during or after prior endocrine therapy. Breast Cancer Res. 2017;19(1):18. doi:10.1186/s13058-017-0807-8
8. Bross PF, Cohen MH, Williams GA, Pazdur R. FDA drug approval summaries: fulvestrant. Oncologist. 2002;7(6):477-480. doi:10.1634/theoncologist.7-6-477
9. Bardia A, Kabos P, Wilks S, et al. Elacestrant, oral selective estrogen receptor degrader (SERD) in patients with ER positive (ER+)/HER2- advanced breast cancer: updated phase 1 efficacy, safety and pharmacodynamic results. Abstract presented at: 2017 San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, TX. Accessed September 22, 2020. https://bit.ly/2FFwSgA
10. Hamilton EP, Oliveira M, Banerji U, et al. A phase I dose escalation and expansion study of the next generation oral SERD AZD9833 in women with ER-positive, HER2-negative advanced breast cancer. J Clin Oncol. 2019;38(suppl 15):1024. doi:10.1200/JCO.2019.38.15_suppl.1024