Smart Watch Used for Biometric Measuring Demonstrates Positive Response, Overall Satisfaction
Investigators anticipate that improved versions of the application will evolve over the phases as patient-specific queries are optimized and as cancer-specific pathways are incorporated. A multidisciplinary team will provide a tailored and integrative approach, concluded the investigators.

A proof-of-concept outlined the feasibility of rapidly implementing a tailored patient monitoring service demonstrated positive responses and overall satisfaction among patients receiving cancer care at a general hospital in the United Kingdom during the COVID-19 pandemic.1
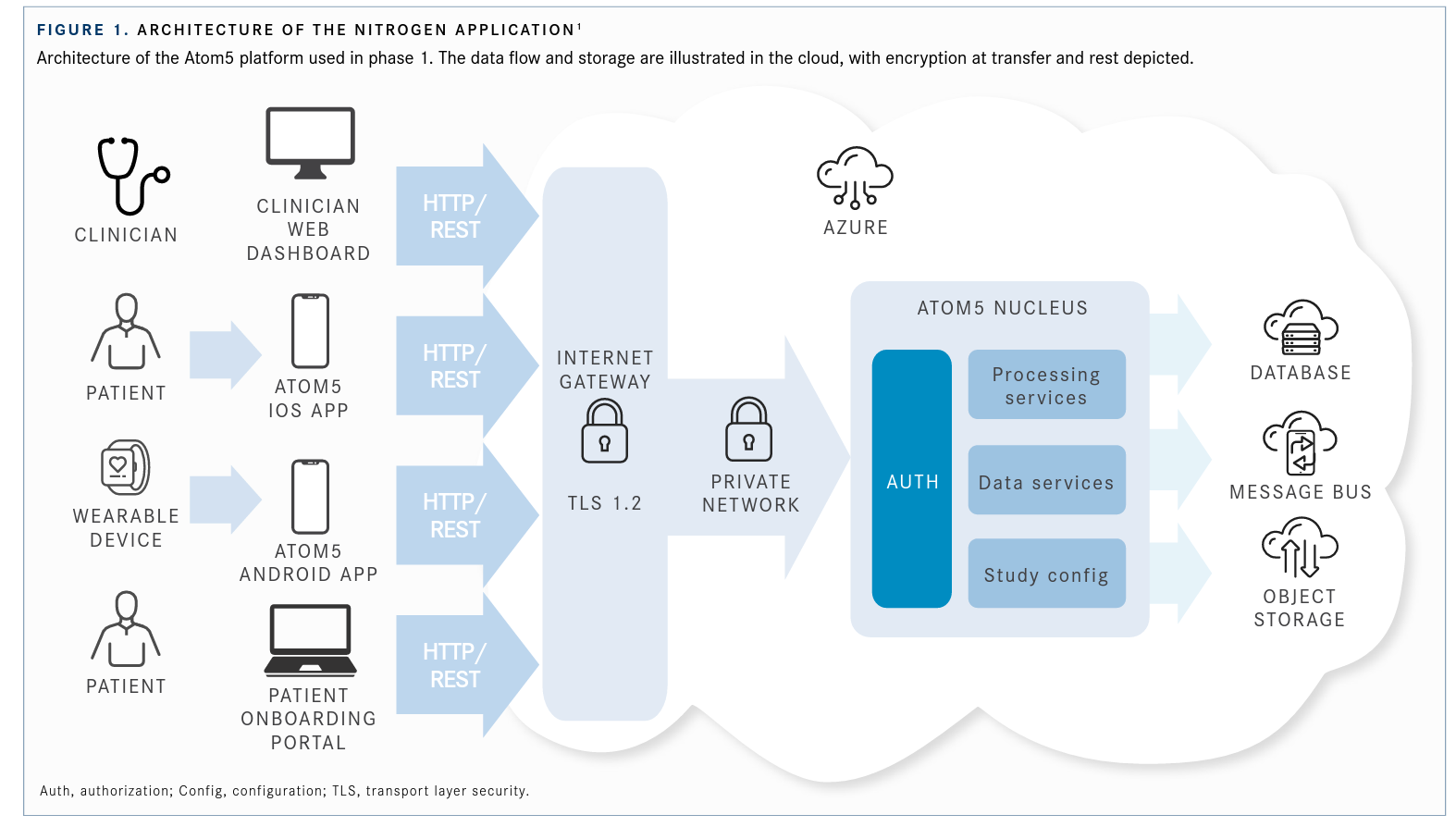
Forty-eight patients were randomized to receive the Garmin vívosmart 4, a lifestyle watch, to wear daily for 2 weeks. The watch recorded heart rate, accelerometer, ambient light, and pulse blood oxygen saturation (Spo2). Investigators deemed the pulse oximeter feature as particularly relevant with regard to COVID-19 symptomatology and remote surveillance. Biometric measurements were collected by an app called Nitrogen, which delivered the data for database storage and services, processing services, and message out-reach (FIGURE 11).
The Nitrogen app presented wearers with daily yes/no questions about new or worsening cough, breathing difficulties, fever, unusual or worse-than-usual fatigue, and general well- being. Patients could also choose the symptoms they were experiencing from a preestablished list, including body aches and chills; nausea; vomiting; loss of appetite, taste, and smell; and abdominal pain. The watch could also provide objective measurements of spot-check Spo2 and continuous heart rate. Any COVID-19–like symptoms were assessed daily for deterioration through the application, and an alert and notification were sent to patients to complete. Clinicians could monitor vital sign measurements from the device and those provided by the patients on a web-based dashboard.
The metrics were monitored by the wrist-band, which was worn day and night for the continuous measurement of heart rate and the nightly assessment of Spo2.
Investigators reported that 40 patients (83%) recorded data, which indicated that the technology was accessible for the majority of patients. Generally, patients demonstrated a high engagement (FIGURE 21) with completing the questions, recording symptoms for a median of 9 days. At week 5, 31 patients were still using the technology, and at week 21, 13 patients were using the device without prompting, reminder, or further intervention from the clinical team.
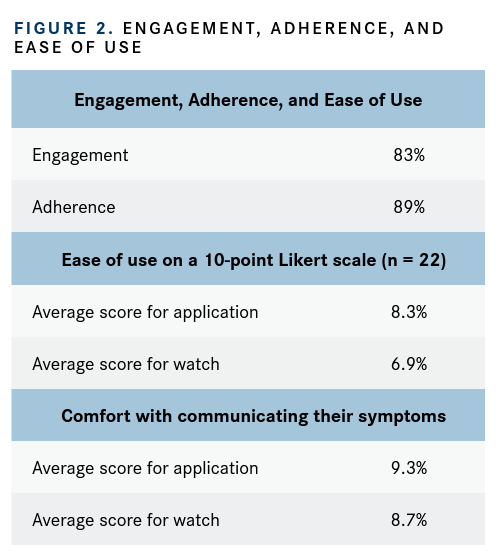
A short feedback survey was implemented in a self-selecting subgroup of 22 patients. Patient feedback revealed overall satisfaction with both the application and the wearable device (FIGURE 2), with levels indicated on a 10-point Likert scale from 0 (not at all) to 10 (very much).
The investigators noted a number of assumptions and observed a number of limitations during implementation, particularly regarding patient information technology literacy. They “assumed that patients knew how to connect to Wi-Fi, navigate an app store, and use Bluetooth connectivity.” Of the 12 patients who had minimal use of the application, the majority struggled to activate the Bluetooth connection on their mobile phone to allow linking the application to their wearable device or mistakenly used the Garmin Connect App, which prevented clinicians from receiving the data on the Nitrogen app. They noted these limitations highlighted a subgroup that would require support.
The investigators’ main concern was finding a balance between a validated minimally invasive device that was easy to use for patients who might have poor eyesight or limited fine motor dexterity.
One finding that encouraged the investigators was that the majority of patients expressed an interest in tracking their own data directly on the application. Although this represented a phase 1 rollout, patients encouraged the development team to incorporate their feedback into phase 2 and further. The investigators believe the addition of this feature to future rollouts will be likely to increase participation length and patient engagement.
According to the investigators, the initiative will involve a 3-phase process. Phase 2 will involve recruiting acutely unwell patients to monitor their progress as inpatient and outpatients. Phase 3 will involve general new patient recruitment, assessment, reliability, and quality checks of the monitoring device with medical-grade applications.
Investigators anticipate that improved versions of the application will evolve over the phases as patient-specific queries are optimized and as cancer-specific pathways are incorporated. A multidisciplinary team will provide a tailored and integrative approach, concluded the investigators.
References:
1. Komarzynski S, Wreglesworth NI, Griffiths D, et al. Embracing change: learnings from implementing multidimensional digital remote monitoring in oncology patients at a district general hospital during the COVID-19 pandemic. JCO Clin Cancer Inform. 2021;5:216-220. doi:10.1200/CCI.20.00136
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More