Osimertinib Continues to Impress With Response and Disease Control Rates in NSCLC
Results from an observational, multicenter study that evaluated osimertinib reveal encouraging real-world efficacy and acceptable safety in patients with EGFR-positive, advanced non–small cell lung cancer.
Results from an observational, multicenter study that evaluated osimertinib (Tagrisso) reveal encouraging real-world efficacy and acceptable safety in patients with EGFR-positive, advanced non–small cell lung cancer (NSCLC).1 Findings were presented during the European Lung Cancer Virtual Congress 2021.
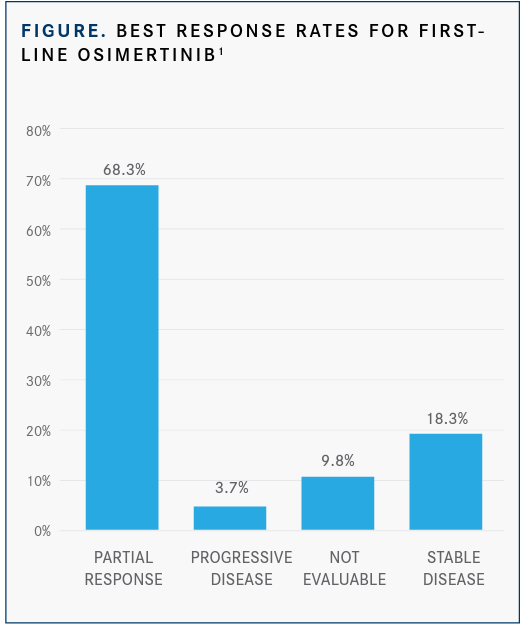
At a median follow-up of 11.2 months, the response rate achieved with the third- generation EGFR tyrosine kinase inhibitor (TKI) was 68.3% and the disease control rate was 86.6% (FIGURE).1 Moreover, the median progression-free survival (PFS) was 22.0 months (95% CI, 11.4-32.5) with the agent, and the median overall survival had not yet been reached. The median time to treatment failure (TTF) was 25.3 months (95% CI, 18.9-not calculable).1
“Osimertinib [has] confirmed efficacy and safety in [the] real world,” lead study author Martina Lorenzi, a medical student in the Department of Surgery at the University of Padua, Italy, and coinvestigators wrote in a poster presenting the data. “[However,] thromboembolic events [with the agent] were observed more frequently than previously reported.”
Real-world data regarding progressive dis-ease pattern and safety of frontline osimertinib in patients with EGFR-mutated, advanced NSCLC are limited. As such, investigators set out to examine this further. Between November 2018 and November 2020, 82 patients with EGFR-mutant locally advanced or metastatic NSCLC receiving first-line treatment with the EGFR TKI were enrolled. Patients received osimertinib until treatment failure.
The primary end points of the research included median PFS, TTF, and progressive disease (PD) patterns. PD was classified as isolated, oligoprogression, and systemic. A key secondary end point of the trial was safety.
The median age of participants was 65 years (range, 30-88), 63.4% were female, 56.1% were never smokers, and the majority (93.9%) had adenocarcinoma. Additionally, 47.6% had EGFR exon 19 deletions, 43.9% had EGFR exon 21 L858R mutations, 7.3% had other EGFR mutations, and 1.2% had unknown baseline EGFR mutation status.
Most patients (95.1%) had stage IV disease at the time of diagnosis, 79.3% had an ECOG performance status of 0 or 1, 93.9% had a Charlson Comorbidity Index score of more than 6, and 63.4% had less than 3 metastatic sites at diag-nosis. Notably, 32.9% had present brain metastases at the time of diagnosis.
Additional results showed that PD was expe-rienced by 34% (n = 28) of patients and that the median number of sites was 2 (range, 1-7). The most frequently observed sites of PD included the lung (67.9%; n = 19), the bone (39.3%; n = 11), and the brain (25.0%; n = 7). New sites of PD were noted in 39.3% (n = 11) of patients, with a median of 1 site (range, 1-4). Moreover, 17.9% (n = 5) of patients experienced isolated PD, 21.4% (n = 6) experienced oligoprogression, and 57.1% (n = 16) experienced systemic progression.
Additionally, a total of 9 cases, at the time of progression, underwent tissue and/or liquid rebiopsy. Five patients were found to have targetable resistance mechanisms; 3 patients (33.3%) had tumors that harbored MET amplification, 1 (11.1%) had MET amplification/EGFR amplification, and 1 (11.1%) had HER2 amplification.
Regarding safety, the most commonly expe-rienced all-grade toxicities with osimertinib included diarrhea (40.2%), rash (39.0%), paronychia (26.8%), and creatinine increase (30.5%). The most common grade 3 or higher adverse effects (AEs) included thromboembolic events (7.3%), diarrhea (4.9%), arterial thromboembo-lism (2.4%), and rash (2.4%).
Additionally, 23.2% of patients experienced an interruption with osimertinib due to toxicities, with 6.1% of patients reporting a permanent interruption. Temporary treatment interruption–related AEs included diarrhea (15.8%), heart failure (10.5%), paronychia (10.5%), rash (15.8%), and neutropenia (10.5%). Toxicities that resulted in permanent interruption of osimertinib were thrombotic events, heart failure, and arterial thromboembolism (20% each).
The study is ongoing, with investigators working to enroll a larger patient population.
REFERENCE
1. Lorenzi M, Dal Maso A, Ferro A, et al. First line (1L) osimertinib in EGFR mutant (mut) advanced non-small-cell lung cancer (aNSCLC) patients (pts): progression (PD) pattern and safety in the real-world (RW). J Thorac Oncol. 2021;16(suppl 4):S779. doi:10.1016/S1556-0864(21)01991-2