Roundtable Discussions: Barriers in Giving Therapies in Relapsed/ Refractory DLBCL
During a Targeted Oncology Case-Based Roundtable event, John M. Burke, MD, discussed the challenges with treating relapsed or refractory diffuse large B-cell lymphoma with a group of peers.
John M. Burke, MD


During a Targeted OncologyTM Case-Based Roundtable event, John M. Burke, MD, associate chair, US Oncology Hematology Research Program at Rocky Mountain Cancer Centers in Aurora, CO, discussed the challenges with treating relapsed or refractory diffuse large B-cell lymphoma (DLBCL) with a group of peers.

BURKE: What factors are going to help you choose what you want to give?
BALARAMAN: I would say for me, in Florida, it’s going to be IPI [International Prognostic Index] and performance status. Those would be my top 2 that I would look at.
BURKE: For good performance status vs not-so-good performance status, how are you going to differentiate your treatment there?
BALARAMAN: If they have a good performance status and…a high score, then I would be looking at CAR [chimeric antigen receptor] T-cell therapy for those patients because I have access to it. If they have a slightly poor performance status but high IPI, and they had either relapsed or refractory [disease] fairly quickly, then for those patients I would still use third-line therapy. I would just be very cognizant of the fact that they probably won’t do very well with it. I’m definitely not sending those patients to get CAR T. I’m keeping them in house. I’ve also been doing fewer and fewer allogeneic stem cell transplants. It’s just something I don’t do with the CAR T coming up [as an option].
FARIDI: I deal with mostly the elderly population, and I am going to look for the comorbidities. Also, my practice is in North Dallas in the Sherman area, [one of the largest areas in the] state, and convenience for the patient is also important [in terms of] how they can access the CAR T and other therapies.
CHALLAGALLA: I still think performance status would be the backbone for any decision-making. With the advent of CAR T, allogeneic stem cell transplant takes a big backseat.
BURKE: [If a patient has] good performance status, what are you going to do?
CHALLAGALLA: I would still look for CAR T if the patient is able to travel. Likewise to Dr Faridi, I practice 2 hours away from the availability of any CAR T or stem cell transplant teams. But still, I think honestly CAR T has evolved into a much easier and safer therapy, so we are not afraid to refer [patients] to CAR T like we were when it first started. Good performance status, still CAR T. Marginal performance status, still get an opinion, but prepare for either tafasitamab [Monjuvi] or any of the other options.
BURKE: Let’s say you have a patient who you don’t feel is a candidate for CAR T, so you’re going to choose from one of the other options. Between those options, how are you making that choice?
CHALLAGALLA: I think [it’s important to consider] the comorbidities and what adverse events [AEs] they had from prior regimens—so use a regimen that has different AEs from previous [ones]. Comorbidities and the prior regimens play a role in that regard.
BURKE: It seems like patients are leaning toward CAR T if they’re eligible. But if they’re not eligible, how [do you choose] between the available options?
MELNYK: [A patient might not be] eligible for CAR T if they didn’t have a complete response to second-line therapy. [If the performance status was not very good], CAR T is what we all want to do because it seems to have the best opportunity for a long-term remission. But otherwise, you’d want to pick a non–cross-resistant regimen as a third line or a fourth line. Then the discussion goes to this is now palliative care rather than curative care, probably. And how does the patient want to proceed? That’s how I would look at the problem.
BURKE: Any thoughts on picking between tafasitamab/ lenalidomide [Revlimid], loncastuximab tesirine [Zynlonta], selinexor [Xpovio], or polatuzumab [Polivy]? Any factors in weighing and helping you make that decision?
MELNYK: Well, you could see whether there was a lot of CD19 expression on the tumor, on rebiopsy, if you had something like that. You could look at a number of factors that might sway you toward one or the other. Honestly, I’m not sure any one of them has a particular advantage, and certainly none of them have been tested head to head. All of them would be reasonable depending on what kind of toxicity profile you wanted to entertain.
For instance, for selinexor, I would be thinking the patient would need a better performance status. Some of the other ones, perhaps worse performance status.
BURKE: You’re looking at toxicity profile largely to help you make that decision.
MELNYK: You’re palliating them now.
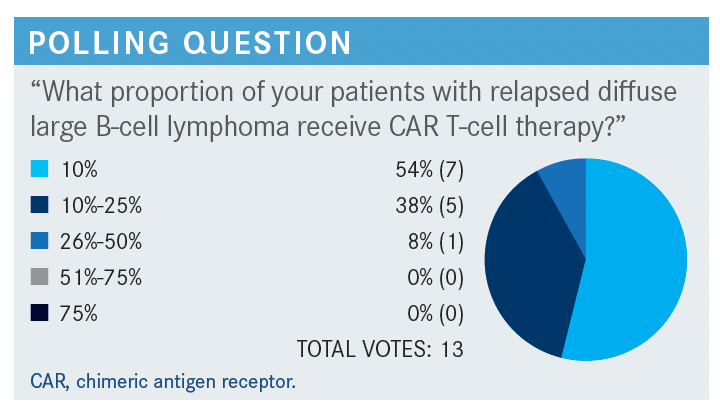
BURKE: Nobody says anything more than 50%. [So we have] relatively low proportions of patients with relapsed DLBCL [diffuse large B-cell lymphoma] receiving CAR T-cell therapy. I think your answers are consistent with what most people think. I read an article…that said that since the approval of commercial CAR T, only about 2000 patients around the country have been treated with commercial CAR T. This was a couple of months ago, so it’s higher than that by now, but still a relatively underutilized treatment modality in patients with relapsed DLBCL.

STONE: Well, we’re fortunate in Plano, Texas, to be fairly close to a transplant center. They also have access to CAR T. Honestly, after a second relapse, I usually send patients to at least be evaluated, and those physicians are the ones that are making the decisions in most cases for either transplant or third-line therapy or CAR T. I personally haven’t had any of my patients receive CAR T, but I have had some go on to autologous stem cell transplant afterward.
Because we have such close access, I often let those physicians make the decision in terms of which therapy would be best in the second relapse. In most cases, I will treat them with second-line therapy if they look like they can tolerate it, if they have a good performance status. But after the second relapse, I would certainly get the opinion of our transplant people.
BURKE: How far away from Plano are your transplant people, drive time?
STONE: It’s really good for us. It’s about a 10- to 15-minute drive from our office.
BURKE: Very convenient.
KURKUL: I will say that it’s fascinating. I’m in Denton, Texas, which is about an hour north of Dallas-Fort Worth if you stretch it. [For] our patients—especially when they are coming from Southern Oklahoma, even at the first relapse—I try to get them in to the city to see our folks at the transplant centers and they do not want to make that travel time. Oftentimes I find myself looking at second-line therapies when I probably would’ve sent them back at the first relapse for an automatic evaluation at that time.
BURKE: Just to elaborate on that, your patients will come to you, and you recommend they go see a transplant doctor in the city, and they’ll say, “I really don’t want to make that drive and deal with that. What else do you have to give me?”
STONE: Pretty much. What can we handle here vs sending you in? I think many of them know it’s not just the evaluation in the city; it’s that it will be a prolonged amount of time.
BURKE: What about CAR T? Do you have the same kind of experience? Say you have a third-line patient, how is that working for you where you’re at in Denton?
STONE: We’re very lucky in that the people in the city… helping us with our autologous/allogeneic decisions are also the ones that have the availability of the CAR T, and they’re very aggressive about getting people into clinical trials if eligible. With CAR T, it’s less whether they’re fit for it [and more] the finance barrier at that point, either due to funding or…lack of available funding for CAR T. For a lot of patients out here, CAR T is barely covered in their expanded oncology insurance.
BURKE: So [there are] some insurance barriers as well there. Dr Gada, how about you in Minnesota? How do things work?
GADA: In Minnesota we are very fortunate because we are very close with people at the University of Minnesota, as well as Mayo Clinic, so access is not a problem. It depends. We’ll refer them at either the first or second relapse, depending on the patient. If I have a younger patient, maybe I’ll do it early sometimes. Then with our collaboration with Mayo Clinic now, we can present our cases at their tumor board too, so that’s the advantage we have also. The patients don’t formally see them but we can still present our cases there. Personally, I’ve not had a patient who’s been eligible for CAR T yet because of comorbidities or other issues. It’s the same group both at the University of Minnesota and Mayo Clinic who do the transplant, as well as CAR T.
BURKE: What’s the travel time for patients from your location to, say, where they get a CAR T or where they get a transplant?
GADA: Mayo Clinic is a little bit of a drive, but maybe an hour and a half or depending on where they live, or a little bit longer. But for the University of Minnesota, for most patients it should be 30 to 40 minutes usually.
BURKE: Do you get the same kind of feedback from some of your patients as Dr Kurkul, where they come in and say, “I don’t want to make that travel; just treat me here.”
GADA: I think if it’s a younger patient [they will go] because we can comanage them. Maybe they’re there for their treatment, but a lot of the follow-ups sometimes we can do at our clinic if we work very closely with them. For an initial consult now, with COVID-19, a lot of telemedicine consults are there, so patients have been willing to at least do a consultation and we comanage patients both from the university as well as Mayo Clinic.
KURKUL: That’s a really valid point. Since the pandemic, one of the few [good] things has been that telemedicine consults have [enabled us to get] a lot of people in that we didn’t see before.
BURKE: It’s almost easier to get them seen [at academic facilities].
BALARAMAN: In Florida, we’re very close to the University of Florida at Gainesville. Then just last week I was told that we [can’t] send any CAR T patients there because they’ve only done 2 infusions of CAR T in the past 2 years, which is a big disappointment for us. So we have to send them away to Tampa, which is Moffitt Cancer Center.
Moffitt has a lag time now of more than 3 to 4 months to be able to harvest cells because they have a shortage. They recommend that we give patients other lines of therapy. A lot of times they’ll tell us: [Use] this line of therapy and then I’ll see them back for CAR T after you get them into some sort of remission. We don’t have good CAR T access at this point.
BURKE: Wow! I’ve never heard of that. Interesting.

FARIDI: [Regarding] the data for loncastuximab, mostly what I heard is [use in] a heavily pretreated patient. So I would still use loncastuximab after I treat with tafasitamab or polatuzumab first. I think that loncastuximab can still show response after that, and I will still use tafasitamab or polatuzumab first because the data are only if the patient was treated with 1 or 2 lines of therapy. Then loncastuximab is mostly for the patients who are treated [with a] median of 3 lines of therapy. I don’t see any barrier. I think it’s very tolerable. Regarding the patients who are not available for CAR T, I think it’s a very good option to treat before CAR T.
MELNYK: My concern is we’re going to have CD19 exhaustion. All these therapies are now acting on CD19, and I think you have to pick and choose which ones you want to use at which times. I’d like more information, more numbers on CAR T after loncastuximab, perhaps even after our other regimens, to see if it really is as good. I suspect it’s not. What’s going to happen is you’re going to have to pick and choose which CD19 approach you want to take and then try to use something else further down the road to reflect responses. But I suspect that’s going to be the limiting factor.
BURKE: You’re a little worried that you might lose some CAR T efficacy if you use loncastuximab pre–CAR T, and you would like some more data to guide you on that. How about using tafasitamab or loncastuximab after CAR T? Let’s say your patient relapses after CAR T.
MELNYK: Doesn’t CAR T resistance often come with CD19 exhaustion [and] you just don’t see it?
BURKE: I don’t know. I know that sometimes the CD19 [targeting] is lost, but there may be other mechanisms of resistance to CAR T other than loss of CD19.
MELNYK: Maybe, but I heard that’s one of the big ones.
BURKE: So you would think about using other alternatives as opposed to hitting CD19 again if you had a relapse after CAR T?
MELNYK: Yes.
BALARAMAN: I’m not so familiar with these drugs because they’re all new, but in my own mind when I saw these approvals coming, I figured I would use loncastuximab in patients who potentially are not eligible for CAR T because I’d be able to hit the CD19 vs tafasitamab. But the big thing for loncastuximab that stands out is the ones that are going to respond are going to respond very quickly. If a patient is very ill and I don’t think I’m going to be able to get them to another line of therapy, then maybe I’m going to try to use loncastuximab. I haven’t used the drug, but that’s how I put it in my mind.
CHALLAGALLA: Can you comment on using loncastuximab as a bridging to CAR T? Because there is some duration by the time the patient is ready as there is a response rate to CAR T post loncastuximab for a brief time. How would it perform as a bridging drug to CAR T?
BURKE: It hasn’t been formally studied as that, but other than the 47%, 7 of 15 patients who went on to get CAR T after loncastuximab had responses.
CHALLAGALLA: But those are probably folks who have relapsed on loncastuximab. Am I right?
BURKE: They could have been, yes. They could’ve progressed on loncastuximab and then [gone] on to get CAR T therapy later. I don’t know that anybody has any data on how it performs as a bridging therapy and what happens with your CAR T after that.
Dr Kurkul, how about you for your patients who have to drive from Southern Oklahoma, comparing loncastuximab with tafasitamab or polatuzumab/bendamustine/rituximab? Any pros and cons you can think of?
KURKUL: I don’t have any experience with loncastuximab. [What was the dosing schedule?]
BURKE: Loncastuximab is every 3 weeks they get an infusion for 30 minutes.
KURKUL: That seems reasonable. There are so many of these drugs coming out and we’re getting told in the community, “Don’t give bendamustine if they’re going to go for a transplant,” or “Don’t give melphalan for this.” It’s really interesting to see something I assumed would deaden CAR T collection. I’d love to know more about that.
BURKE: What about if you want to [give] a patient therapy next week and you have to order the lenalidomide with the tafasitamab? Has anybody done that? How is that working? Does it take longer than a week to get lenalidomide going? How does that work in your practices?
KURKUL: Oral drugs are some of the hardest to get going quickly, period. I don’t think I could get it. I’m also curious how many folks need a dose reduction on the lenalidomide; 25 mg for most of my elderly [patients] would be a hefty kick.
BURKE: It’s a common comment, that you look at your 80-year-old, 110-lb patient and [think], “Do I really want to give this person 25 mg of lenalidomide?”
FARIDI: Whenever I try to put the lenalidomide dose of 25 mg on my patients, next day I get the call from the pharmacy: The patient’s glomerular filtration rate [GFR] is 30 mL/min, a 75-year-old. Do you think that patient needs to have a dose reduction? It’s hard to give 25 mg to elderly folks.
BURKE: Have you used this combination and did you reduce the lenalidomide dose or…go with the 25 mg.
FARIDI: No. I’m talking about the other diseases such as multiple myeloma; we use lenalidomide all the time. We have to always dose reduce. These elder folks, most of them have low creatinine clearance and low GFR, so [regarding a] 25-mg dose, I think 70% of people are able to keep it. In my population, it probably would be less than 30% [who would] go and get the full dose of 25 mg.
BURKE: Does…the weekly schedule of tafasitamab for 3 months and then every other month vs an every-3-week schedule of loncastuximab make [anyone] lean toward one over the other? Or is it not a big deal for patients to come in for the tafasitamab?
SHAO: I’m in Portland, Oregon, so most of my patients are not very far away, so it’s doable either way.
BURKE: I see. Not a big factor. What are your thoughts on polatuzumab/bendamustine/rituximab; have you used it? Based on the data you’ve seen, what do you think about picking that vs a CD19-targeted therapy?
SHAO: I have very few of these patients. I guess I’m very lucky most of my patients are young. If they have relapsed disease, they would’ve received a transplant. But I am impressed with the tafasitamab/lenalidomide data when we see a progression-free survival [rate] of 50% at 12 months or 16 months or what have you. That’s very impressive. That’s comparable to transplant data. The next time I see that patient who’s not transplant eligible, that’s probably the program I will reach for.
REFERENCE
Caimi PF, Ai WZ, Alderuccio JP, et al. Efficacy and safety of loncastuximab tesirine (ADCT-402) in relapsed/refractory diffuse large b-cell lymphoma. Blood. 2020;136(suppl 1):35-37. doi:10.1182/blood-2020-137524
