Roundtable Discussion: Jakubowiak Looks at New Agents for Relapsed/Refractory Multiple Myeloma
During a Targeted Oncology Case-Base Roundtable event, Andrzej Jakubowiak, MD, PhD, led a discussion on new agents for the treatment of multiple myeloma.
Andrzej Jakubowiak, MD, PhD

Participant List
During a Targeted Oncology Case-Base Roundtable event, Andrzej Jakubowiak, MD, PhD, led a discussion on new agents for the treatment of multiple myeloma.
Case summary
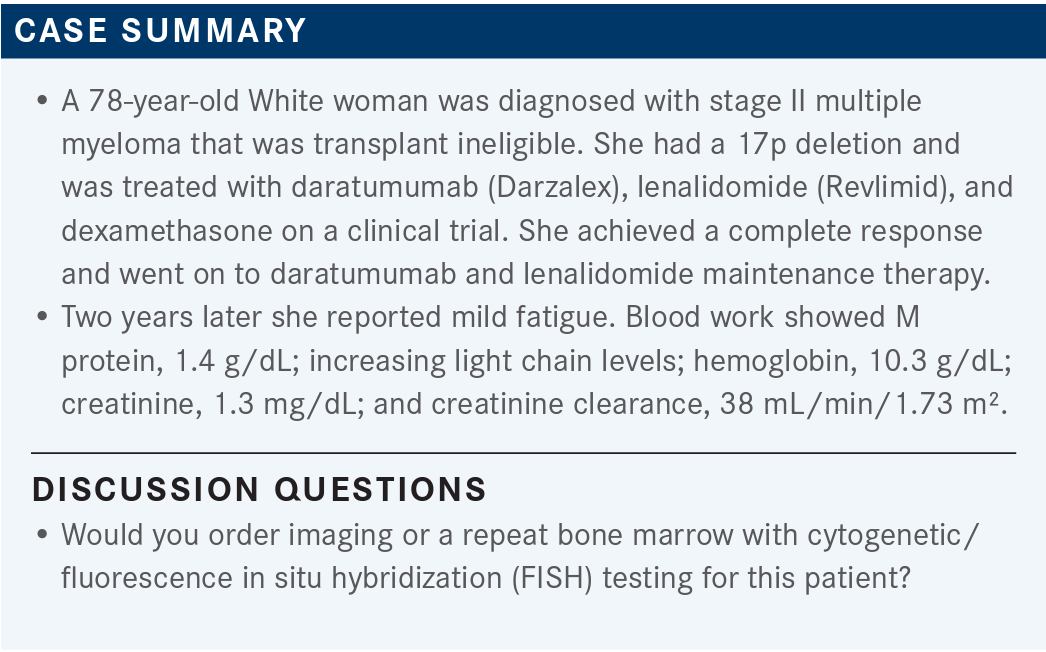
JAKUBOWIAK: The patient was diagnosed initially with 17p, which tends to behave more aggressively, sometimes out of proportion to increase in M spike. I think positron emission tomography [PET] imaging would be reasonable. How about repeating the bone marrow biopsy and checking cytogenetic FISH?
KOKO: What would be the benefit of a repeat bone marrow biopsy? We already know the patient is high risk; she has 17p deletion, 1 of the highest-risk genetic abnormalities, so I wouldn’t repeat the bone marrow biopsy.
GRINBLATT: If you start a new therapy, you want to know whether that new therapy is working. The reason to restage may not be to make sure the patient has progressed—because we know they’ve progressed—but rather to serve as a new baseline because if you give a new therapy, you may need a bone marrow in the future to see how well they’ve responded.
Discussion question

GIMELFARB: I personally don’t think I’d trigger [second-line therapy] because of an increase in M protein but more of a trend. If I see a trend, then it’s concerning, and we start worrying about it. I would also do a PET scan to look for something like plasmacytoma that could cause a problem and be easily treated. Sometimes marrow shows more than the blood shows. I will sometimes do the marrow if I’m on the fence about whether to start treatment or not.
GRINBLATT: If you’re treating someone with an active therapy and their disease meets progression criteria—their M protein has gone up by more than 25%—I would favor changing therapy, even if you don’t have a clinical trial. Drugs have costs and toxicities, so to give someone a therapy while they’re clearly progressing through disease [doesn’t make sense to me].
JAKUBOWIAK: There is a trend among some myeloma leaders to start the next-line therapy, for example, with the reappearance of minimal residual disease [MRD] or if the patient was previously in complete remission and has no change in MRD spike, but there is a reappearance of disease in the bone marrow. That is not our practice. I am, with few exceptions, following the rule that at that point intervention is not indicated, but I suspect that will change over time.
JOSHI: The patient has been on maintenance for 2 years. It’s the rapidity of the rise [of disease] that I look at: Is it a slow rise or rapid rise? I don’t want to burn a bridge; my concern is that we could run out of things to give someone. If somebody is tolerating something really well, you hate to go away from that. Obviously, if there is disease progression.…I do a full workup, including the imaging and the bone marrow, just to see where we are and not just look at the M spike and trends. If it looks like a real progression, obviously go to the next line rather than giving something through which their disease is overtly progressing.
Discussion Question

JAKUBOWIAK: I have to say that I prefer to have a discussion with a newly diagnosed patient about picking the best therapy rather than a discussion [with a patient about] relapsed/refractory disease. It’s more challenging, and I don’t think we have absolute guidelines.1,2 Performance status, cytogenetics, time and extent of relapse, and other factors affect what we choose for the patient.
For some of us, including myself, the golden rule in oncology, in the absence of specific guidance based on the cytogenetics or the biology of the disease, is that if the patient’s disease is progressing through the current treatment, [we should] change to 1 or preferably 2 different agents if we can apply them. A lot of data show that a triplet regimen could be even better; of course, we will still look at prior toxicity, the status of the patient, and other factors.
KOKO: This patient may be in the initial phase of progression. Is it reasonable to increase the frequency of daratumumab and continue the treatment rather than change it altogether?
JAKUBOWIAK: There was a time when we were just starting daratumumab that we were tempted to reintroduce more frequent daratumumab, [and do the same] with lenalidomide maintenance. I think data supports that the more effective way of addressing progression of the disease, regardless of whether we are using optimal dosage or not on a given regimen, is to switch to different agents and different therapy. But nothing is absolutely written in stone.
GIMELFARB: It sounds like this patient hasn’t had proteasome inhibitor in a while—or ever. And so that would be a backbone of my next regimen.
NABRINSKY: I’m not sure I would keep daratumumab, but I would probably use carfilzomib [Kyprolis]- and pomalidomide [Pomalyst]-based therapy.
Polling Questions
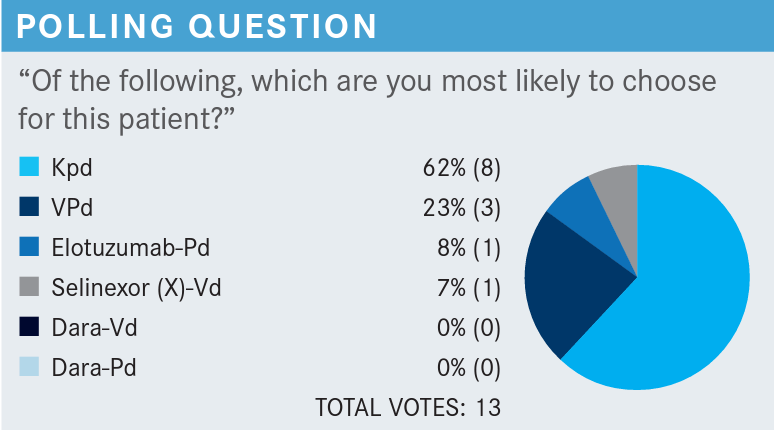
JAKUBOWIAK: We’re switching from [lenalidomide to pomalidomide] and adding a proteasome inhibitor. Pomalidomide, in some studies, has been shown to be active in a P53 deleted subset of patients. A lenalidomide and dexamethasone study showed that and a couple of other studies with pomalidomide as well, although not randomized trials. There are a couple of other preferred choices. VPd is very comparable. In fact, in direct comparison, those 2 adjuvant therapies are in the same ballpark of responses and progression-free survival.
Discussion Question

JAKUBOWIAK: Selinexor is one of the few drugs for which we have proof of a completely different mechanism of action.3-5 It inhibits exportin, one of the mechanisms of export of oncogenes from the nucleus, and allows the immune system of a patient, especially those with deficient P53, to engage in apoptotic pathways and eliminate more cancer cells. I think that activity of this combination is well supported. Toxicity is usually well managed. Are you comfortable using selinexor in the first and second line of therapy?
GIMELFARB: The majority of us who have not had a patient in a clinical trial for the first-line therapy haven’t used daratumumab up front for very long. And so most of our second-line therapy has been daratumumab-based. A lot of us haven’t gotten to a selinexor line because of that. But now that daratumumab has moved up to firstline therapy in so many patients, I think that’s probably going to change.
JAKUBOWIAK: I agree. The landscape is changing dramatically. [Comparing older studies to new ones may be unfair because] older studies may not have been able to generate results as impressive as ours. For example, the results of POLLUX were excellent and just beyond anybody’s expectations.6
Discussion Question

GIMELFARB: Most of my patients who need secondline therapy are daratumumab naïve. So I tend to use daratumumab with either pomalidomide or carfilzomib, depending on what they had before, with success. I think daratumumab is a great backbone. Lenalidomide has been used first line, for the most part, so daratumumab, pomalidomide, or some combination of that would be my second line for most patients.
GRINBLATT: Many patients who have experienced relapse have had a transplant and are on lenalidomide maintenance. They’ve had bortezomib [Velcade], lenalidomide, and dexamethasone as their initial therapy. I usually use daratumumab, pomalidomide, and dexamethasone in that setting.
HADDAD: I also consider the type of progression. Is it slow progression? Is it more aggressive progression with organ damage? I try to make that distinction. Also, how long has the patient been on the first-line treatment? Is the disease truly refractory, or do we need to reinstitute chemotherapy drugs we used in the earlier line? I look at all that as well.
JAKUBOWIAK: How important for you is switching to a different mechanism of action?
CHENNAMANENI: I think it’s important to change to a different mechanism of action in the second or third line. Usually, 2 different drugs or 2 different mechanisms will give us better results.
Discussion Question

NABRINSKY: The main changes in my practice are using more daratumumab and more pomalidomide. I stopped using elotuzumab [Empliciti] altogether.
JAKUBOWIAK: There has been evolution in the regimens that we use. There was a period when carfilzomib, lenalidomide, and dexamethasone was most used. We had too many patients progressing on lenalidomide, and then the pomalidomide combination was approved. Daratumumab, pomalidomide, and dexamethasone was probably the most common regimen for about a year and a half.
In the situation where you have very good long-standing response and a patient’s disease is progressing on lenalidomide, my top 3 choices will be daratumumab, pomalidomide, and dexamethasone; carfilzomib, pomalidomide, and dexamethasone; and isatuximab [Sarclisa], pomalidomide, and dexamethasone. All of these, I would say, may have comparable response rates and duration of response. We have been kind of frustrated that we have to make choices between these 3. We [last presented data on treating this patient population] at the American Society of Hematology 2020 Annual Meeting. There are a small number of patients on the trial, just over 20 patients, but our patient population is rather tough. Over 50% of the patients achieved stringent complete response, which is remarkable, in my opinion and the tolerability is good. This is still an actively enrolling study and with this triplet combination, there is good potential to not miss out on the best choice of drug because we don’t know enough about the biology of the disease.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 7.2021. Accessed July 13, 2021. https://bit.ly/36TOWx6
2. Dimopoulos MA, Moreau P, Terpos E, et al; EHA Guidelines Committee, ESMO Guidelines Committee. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(3):309-322. doi:10.1016/j.annonc.2020.11.014
3. Xpovio. Prescribing information. Karyopharm Therapeutics Inc; 2020. Accessed July 14, 2021. https://bit.ly/3eG0OqV
4. FDA approves selinexor for refractory or relapsed multiple myeloma. Food and Drug Administration. Updated December 18, 2020. Accessed July 14, 2021. https://bit.ly/2UX4AFq
5. Grosicki S, Simonova M, Spicka I, et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. Lancet. 2020;396(10262):1563-1573. doi:10.1016/ S0140-6736(20)32292-3
6. Dimopoulos MA, Oriol A, Nahi H, et al; POLLUX Investigators. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319-1331. doi:10.1056/NEJMoa1607751

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More